1. Definition
What is a Rongeur (Kerrison/Lempert)?
A Kerrison Rongeur, often referred to as a Lempert Rongeur in some contexts, is a specialized, precision surgical instrument designed for the meticulous removal of bone. It is a staple in surgeries requiring delicate bone work near sensitive neural or vascular structures. Unlike a general bone rongeur, which crushes or bites, the Kerrison functions more like a “punch” or “punch forceps.” Its primary function is to cleanly excise small, precise sections of bone, creating an opening (a “foraminotomy” or “laminotomy”) to relieve pressure on nerves or to access deeper surgical sites.
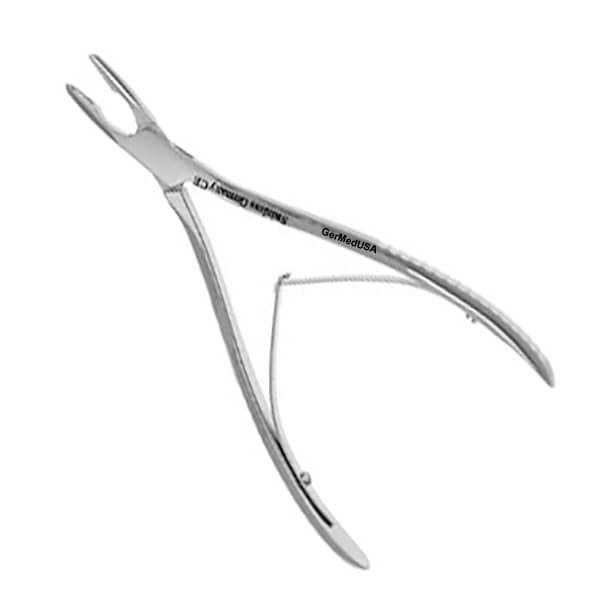
Primary Function: To safely and accurately remove small pieces of bone in confined, critical anatomical areas (e.g., spinal canal, skull base) with minimal trauma to surrounding soft tissues.
How it Works
The Kerrison rongeur operates on a simple yet ingenious mechanical principle:
- The “Footplate”: The lower jaw of the instrument is a thin, flat, and often angled plate that is carefully slid between the bone to be removed and the underlying delicate tissue (like the dura mater covering the spinal cord or a nerve root).
- The “Bite”: The upper jaw, which contains a sharp, rectangular or oval-shaped cutting window, is then compressed by the surgeon’s hand.
- The “Punch”: As the upper jaw closes, its sharp edges shear off the bone that is positioned within the window, while the footplate acts as a physical barrier, protecting the vital structure beneath it. The excised bone fragment is retained within the jaw window.
Key Components
- Handles: Often ring handles with a spring mechanism. The spring keeps the jaws open for positioning, and squeezing the handles closes the jaws to take a bite. Ergonomically designed handles reduce surgeon fatigue.
- Shaft/Shank: Connects the handles to the working end. It can be straight, angled (45°, 90°), or bayoneted. The angle determines the approach and line of sight, crucial for deep or angled surgical corridors.
- Upper Jaw (Cutting Window): Contains the sharp, precision-ground cutting edges that define the size and shape of the bone bite (e.g., 1mm x 2mm, 2mm x 3mm, 3mm x 5mm).
- Lower Jaw (Footplate): The protective, flat plate that guards underlying tissues. Its length, width, and angle are matched to the upper jaw.
- Ratchet/Lock: Some models feature a ratchet mechanism to lock the jaws closed on a piece of bone for removal from the surgical field.
- Depth Gauge Markings: Some premium models have markings on the footplate to indicate the depth of insertion.
2. Uses
Clinical Applications
- Spinal Surgery (Most Common):
- Laminectomy/Laminotomy: Removing part of the lamina (roof of the vertebral bone) to decompress the spinal cord or nerve roots in conditions like spinal stenosis, herniated discs, or tumors.
- Foraminotomy: Enlarging the neural foramen (the hole where the nerve root exits the spine) to relieve nerve root compression.
- Facetectomy: Trimming the facet joints.
- Neurosurgery:
- Craniectomy/Craniotomy: Shaping bone flaps or removing skull bone near the brain, sinuses, or cranial nerves.
- Microvascular Decompression: Removing tiny bits of bone around cranial nerves (e.g., trigeminal nerve).
- Otolaryngology (ENT):
- Mastoidectomy: Removing bone from the mastoid process during ear surgery.
- Ethmoidectomy: Working in the delicate ethmoid sinus bones.
- Orthopedic Surgery: Used in small bone surgery of the hand, foot, or for meticulous work in joint arthroplasty.
Who Uses It
Primarily used by surgeons: Neurosurgeons, Spinal Surgeons, Orthopedic Surgeons, and Otolaryngologists (ENT surgeons). Surgical Technicians/Scrub Nurses are responsible for passing, maintaining, and cleaning the instrument during surgery.
Departments/Settings
- Operating Rooms (OR) in hospitals and ambulatory surgical centers.
- Specialized departments: Neurosurgery OR, Orthopedic Spine OR, ENT OR.
3. Technical Specs
Typical Specifications
- Bite Size: Defined by the window dimensions (Width x Depth). Common sizes range from 1mm x 2mm (for ultra-fine work) to 5mm x 7mm (for larger bone removal).
- Working Length: Shaft length from the hinge to the tip, typically ranging from 6 inches (150mm) to 9.5 inches (240mm) for deeper access.
- Angulation: The angle of the tip relative to the shaft: Straight (0°), Up-Angled (40°, 45°), Down-Angled, or Bayoneted (90°).
- Tip Configuration: Up-biting, down-biting, or side-biting.
Variants & Sizes
- Kerrison Rongeur: The standard name for the spinal/neurosurgical punch.
- Lempert Rongeur: Often a specific subtype with a distinct, slightly heavier design, traditionally associated with ENT/mastoid surgery, but the terms are frequently used interchangeably.
- Variants are defined by:
- Angulation: Straight, 40°, 90°.
- Bite Size: Multiple sizes are always available on a surgical tray.
- Footplate Length: Short or long footplates for different tissue depths.
Materials & Features
- Materials: Almost exclusively made from medical-grade stainless steel (AISI 410, 420, 440C) for durability, corrosion resistance, and the ability to hold a sharp edge. High-end instruments may use tungsten carbide inserts on the cutting jaw edges for extreme hardness and longevity.
- Special Features:
- Tungsten Carbide (TC) Inserts: Gold-colored jaws that stay sharp 5-10x longer than standard steel.
- Ergonomic Handle Design: Knurled, textured, or contoured handles for non-slip grip.
- Single-Piece Forging: For superior strength and durability compared to welded joints.
- Matte/Black Finish: Reduces glare under bright OR lights.
Models
Models are generally categorized by manufacturer series rather than distinct model names (e.g., Aesculap TwinPower Kerrison, Scanlan Kerrison Punch, B. Braun Aesculap Kerrison). The specification (e.g., “40° Up-Biting, 2x3mm, 7″ Shaft”) is the critical identifier.
4. Benefits & Risks
Advantages
- Precision & Safety: The footplate is the defining safety feature, allowing bone removal directly over delicate tissues.
- Control: Allows for meticulous, incremental bone removal.
- Versatility: Available in configurations for nearly every surgical angle and depth.
- Tissue Preservation: Minimizes trauma to adjacent muscles, nerves, and dura.
Limitations
- Small Bite Size: Removing large volumes of bone can be time-consuming.
- Mitigation: Used in conjunction with high-speed drills (for bulk removal) and then the Kerrison for fine, finishing work near critical structures.
- Potential for Jamming: Small bone fragments can jam the mechanism.
- Fatigue: Repetitive squeezing can lead to surgeon hand fatigue, especially in long cases.
Safety Concerns & Warnings
- Dural Tear/CSF Leak: The most significant risk. If the footplate is not properly positioned or slips, the dura (covering the spinal cord/brain) can be torn.
- Nerve Root/Vascular Injury: Similar risk from misplacement or slippage.
- Instrument Failure: Tip breakage or hinge failure, though rare, can occur if excessive force is used or if the instrument is worn.
- Precautions:
- Always visually confirm footplate placement before biting.
- Never force the instrument. If resistance is met, reposition.
- Inspect the instrument for damage (loose hinges, dull or chipped edges) before and after use.
Contraindications
There is no absolute patient contraindication. Its use is technically contraindicated if the bone is too thick or dense for the selected bite size, as this can lead to instrument damage or slippage. The surgeon must select the appropriate tool (e.g., drill, larger rongeur) for the task.
5. Regulation
The Kerrison Rongeur is classified as a surgical instrument.
- FDA Class: Class I (Exempt from premarket notification [510(k)] under regulation 21 CFR 872.4120 – “Bone Cutting and Rongeur Instrument”). It is subject to general controls.
- EU MDR Class: Class I (Rule 1 – Non-invasive instruments for cutting). Requires CE marking under the EU MDR.
- CDSCO Category: Class A (Low-risk) medical device under the Medical Device Rules, 2017 in India.
- PMDA (Japan): Generally classified as a Class I “Medical Device for General Use” (一般医療機器).
- ISO/IEC Standards:
- ISO 13485: Quality Management Systems for medical device manufacturing.
- ISO 7153-1: Materials for surgical instruments – Part 1: Metals.
- IEC 62366-1: Application of usability engineering to medical devices.
6. Maintenance
Cleaning & Sterilization
- Point-of-Use Care: Wipe during surgery to prevent bioburden drying. Do not soak in saline, which can cause corrosion.
- Cleaning: Immediate post-op cleaning with enzymatic detergents and neutral pH solutions. Use soft brushes; ultrasonic cleaning is highly effective for the hinge and jaw mechanisms. Always clean with jaws open.
- Sterilization: Autoclave (Steam Sterilization) is the standard method. Use wrap or cassette systems. Follow manufacturer’s recommended cycles (typically 132-135°C for 3-10 minutes in a prevacuum autoclave).
Reprocessing
Standard sterilization is sufficient as it is a critical device (enters sterile tissue). It is not designed for single-use; reprocessing is mandatory between patients.
Calibration
Does not require electronic calibration. However, regular functional inspection is vital:
- Check for alignment of jaws; they must meet evenly.
- Check for sharpness. A dull rongeur requires excessive force and is dangerous.
- Check hinge for smooth action and any play or wobble.
- Check for cracks, chips, or corrosion.
Storage
- Store in a dry, climate-controlled environment.
- Use protective tip guards if provided.
- Store in perforated trays or cassettes that allow for complete drying and avoid instrument contact that can damage cutting edges.
7. Procurement Guide
How to Select the Device
- Surgeon Preference: The primary factor. Handle feel, balance, and “action” are highly subjective.
- Surgical Specialty: ENT departments may prefer slightly different designs (Lempert style) compared to spine surgeons.
- Common Sizes/Angles: Purchase the most frequently used configurations (e.g., 2mm, 3mm, 4mm bites in 40° and 90° angles).
Quality Factors
- Feel & Balance: Instrument should feel like an extension of the hand.
- Smoothness of Action: The jaw should open and close with consistent, smooth resistance.
- Cutting Sharpness: Test on a plastic bone model or a specially designed testing material.
- Durability: Look for single-piece forged shafts and robust hinge pins.
- Finish: A uniform, smooth finish resists corrosion and is easier to clean.
Certifications
Look for instruments from manufacturers with ISO 13485 certification. The product should carry CE Marking (for EU) and be registered with the relevant national authority (e.g., FDA Establishment Registration).
Compatibility
Standalone manual instrument. Compatibility relates to tray organization within standardized surgical instrument sets (e.g., lumbar laminectomy set, cervical set).
Typical Pricing Range
- Standard Stainless Steel: $150 – $400 per instrument.
- Tungsten Carbide (TC) Inserts: $400 – $800+ per instrument.
- Note: Usually purchased in sets of 6-12 instruments covering various sizes/angles. A full set can cost $2,000 – $8,000+.
8. Top 10 Manufacturers (Worldwide)
- B. Braun (Aesculap) – Germany / USA. A global leader in surgical instruments. Renowned for their “TwinPower” line of rongeurs, known for durability and precision.
- Johnson & Johnson (Ethicon / DePuy Synthes) – USA / Global. Offers a comprehensive range of instruments for spine and neuro surgery under the Codman and Synthes brands.
- Medtronic – Ireland / USA. Through its surgical technologies division, provides high-quality rongeurs, often integrated with its navigation and imaging systems.
- Scanlan International – USA. Specializes in high-end cardiovascular and specialty surgical instruments, with a strong reputation among neuro and spine surgeons.
- Stryker – USA. A major player in orthopedics and neurosurgery, offering a full portfolio of surgical instruments, including Kerrison rongeurs.
- KLS Martin Group – Germany. Known for innovative, high-precision instruments in maxillofacial, neurosurgery, and ENT. Offers excellent quality.
- Integra LifeSciences – USA. Particularly strong in neurosurgery and reconstructive surgery, with a reliable instrument line.
- Becton, Dickinson (BD) – USA. Through its BD Interventional segment, offers surgical instruments.
- Symmetry Surgical – USA. Manufactures a broad range of surgical instruments, including the popular “Padgett” line.
- Rudolf Medical GmbH – Germany. A respected manufacturer of fine surgical instruments with a long history.
9. Top 10 Exporting Countries (Latest Year – Based on HS Code 901890 Data for Surgical Instruments)
Note: Specific data for Kerrison rongeurs is aggregated under broader categories. The following list reflects leading exporters of surgical instruments, which includes these devices.
- Germany: The global leader in high-precision surgical instruments. Known for superior engineering and quality. Dominates the premium market.
- United States: Major producer and consumer. Home to many leading OEMs (J&J, Medtronic, Stryker).
- Pakistan (Sialkot): The world’s largest center for hand-forged surgical instruments. Produces a vast quantity of mid-range and high-quality instruments for global brands.
- China: Rapidly growing exporter, offering a wide range from low-cost to increasingly mid-tier quality instruments.
- Switzerland: Home to specialized, ultra-high-precision manufacturers, often in the microsurgery segment.
- Japan: Exports high-quality, technologically advanced surgical tools.
- France: Has a strong tradition of surgical instrument manufacturing.
- United Kingdom: Hosts several specialist manufacturers.
- Italy: Known for design and quality in specific surgical domains.
- Netherlands: A significant trade hub for medical devices in Europe.
10. Market Trends
- Current Global Trends:
- Rising Minimally Invasive Surgery (MIS): Demand for longer, narrower, and bayoneted rongeurs that can work through tubular retractors.
- Focus on Ergonomics: Design innovations to reduce surgeon fatigue and risk of Repetitive Strain Injury (RSI).
- Cost Pressure: Hospitals balance purchasing premium, long-lasting instruments (like TC) against lower upfront costs, driving value analysis.
- New Technologies:
- Advanced Materials: Coatings to enhance durability and corrosion resistance (e.g., Diamond-Like Carbon – DLC).
- Enhanced Visualization: Integration with intraoperative imaging/navigation, though the instrument itself remains manual.
- Single-Use/Disposable Rongeurs: Emerging due to concerns about reprocessing quality and Prion disease transmission (CJD), though cost and environmental impact are barriers.
- Demand Drivers:
- Aging global population → increased incidence of degenerative spine disease.
- Growing access to surgical care in emerging economies.
- Advancements in surgical techniques requiring more specialized tools.
- Future Insights:
- Continued growth aligned with spine surgery volumes.
- Smart instruments with embedded sensors (for force feedback) are in early R&D but not yet mainstream for basic instruments like rongeurs.
- The core design will remain unchanged, but materials and manufacturing precision will continue to improve.
11. Training
Required Competency
- Surgeons: Acquired during neurosurgical or orthopedic residency. Competency includes understanding the anatomy, selecting the correct size/angle, proper positioning of the footplate, and applying controlled force.
- OR Staff: Must know proper handling, passing technique, and intraoperative cleaning to prevent jamming.
Common User Errors
- “Blind Biting”: Taking a bite without directly visualizing the footplate’s relationship to the underlying tissue. Never do this.
- Using the Wrong Size: Using a 1mm bite on thick cortical bone, causing jamming or instrument strain.
- Leveraging: Using the instrument as a lever to pry bone, which can break the tip.
- Inadequate Maintenance: Using a dull or misaligned instrument, requiring excessive force and increasing risk.
Best-Practice Tips
- Sequence: Use a drill or osteotome to create a starting hole and thin the bone before using the Kerrison for final removal.
- Open Fully: Open the jaws completely before repositioning for each new bite to ensure clean cuts.
- Clear Fragments: Frequently clear bone fragments from the jaw window to prevent jamming.
- Two-Handed Control: In delicate areas, use one hand to stabilize the shaft and the other to squeeze, maximizing control.
12. FAQs
- Q: What’s the difference between a Kerrison and a Lempert Rongeur?
- A: Historically, the Lempert had a specific design for mastoid surgery. Today, the names are often used interchangeably in many hospitals, especially for spinal surgery. The most important identifier is the bite size and angle.
- Q: Why did my Kerrison rongeur jam?
- A: Usually due to taking too large or dense a bone bite, or not clearing previous fragments. Soaking in sterile water or enzymatic cleaner during surgery can help loosen debris.
- Q: How often should Kerrison rongeurs be sharpened?
- A: When dullness is detected during pre-op inspection. Frequency depends on use. TC-edged instruments rarely need sharpening. Standard steel instruments may need it after 20-50 uses.
- Q: Can a Kerrison be used on soft bone (e.g., in osteoporosis)?
- A: Yes, but with extreme caution. The footplate can easily crush through soft bone and injure underlying tissue. Smaller bites and lighter pressure are essential.
- Q: Is it better to buy single or double-action rongeurs?
- A: Kerrison/Lempert rongeurs are almost exclusively single-action (one moving jaw). Double-action rongeurs (both jaws move) are a different type of instrument for different purposes.
- Q: What does “up-biting” and “down-biting” mean?
- A: It refers to the direction the cutting window faces relative to the footplate. An “up-biting” (the most common) removes bone that is above the footplate level. A “down-biting” removes bone below.
- Q: Are disposable Kerrison rongeurs effective?
- A: They guarantee sharpness and sterility and eliminate reprocessing costs/errors. However, their feel and balance are often inferior to high-quality reusable ones, and they generate medical waste. They are a situational tool.
- Q: How do I choose between a 40° and a 90° angle?
- A: 40° (or 45°): The workhorse for most laminectomies, offering a good line-of-sight. 90° (Bayoneted): Essential for deep cavities or MIS procedures where your hand is out of the line of sight (like looking down a tube).
13. Conclusion
The Kerrison (Lempert) rongeur is an indispensable, precision manual instrument in the surgeon’s armamentarium, particularly in spinal and neurological surgery. Its defining safety feature—the protective footplate—allows for bone removal in the most critical anatomical locations. Success with this tool hinges on a deep understanding of its mechanics, selecting the correct size and angle for the task, meticulous surgical technique focused on footplate placement, and a rigorous institutional commitment to proper maintenance and inspection. While technological advances continue in the surgical field, the fundamental design and utility of the Kerrison rongeur ensure it will remain a cornerstone of operative suites worldwide for the foreseeable future.
14. References
- Rengachary, S. S., & Ellenbogen, R. G. (Eds.). (2005). Principles of Neurosurgery (2nd ed.). Elsevier Mosby. (Chapter on Surgical Instruments).
- Benzel, E. C. (2017). Spine Surgery: Techniques, Complication Avoidance, and Management (4th ed.). Elsevier.
- Association of periOperative Registered Nurses (AORN). (2023). Guidelines for Perioperative Practice. (Sections on Instrument Cleaning and Sterilization).
- U.S. Food and Drug Administration (FDA). (2023). Code of Federal Regulations Title 21, Sec. 872.4120. https://www.accessdata.fda.gov/
- European Commission. (2017). Regulation (EU) 2017/745 on medical devices (MDR).
- International Organization for Standardization (ISO). ISO 13485:2016 Medical devices — Quality management systems — Requirements for regulatory purposes.
- Instrument Manufacturers’ Technical Data Sheets (Aesculap, Scanlan, KLS Martin).
- World Bank & International Trade Centre Trade Map Data. (2023). Analysis of HS Code 901890 exports.