1. Definition
What is a Manual Defibrillator?
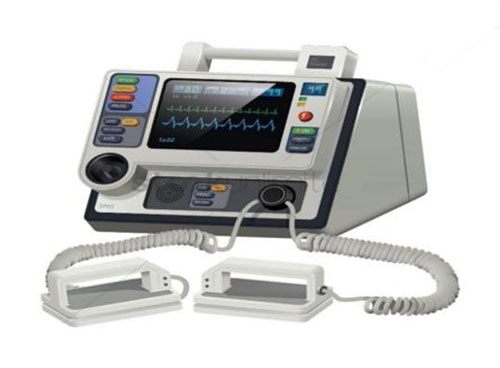
A manual defibrillator is a sophisticated, clinician-operated medical device designed to deliver a therapeutic dose of electrical energy (a “shock”) to the heart of a patient experiencing a life-threatening cardiac arrhythmia, primarily ventricular fibrillation (VF) or pulseless ventricular tachycardia (VT). Unlike Automated External Defibrillators (AEDs), which analyze the heart rhythm and decide if a shock is needed automatically, manual defibrillators require the operator—a trained healthcare professional—to interpret the cardiac rhythm on a display and manually make the decision to charge and deliver a shock. They are the definitive tool for advanced cardiac life support (ACLS) in hospital settings.
How it Works
The core principle is electrical cardioversion. In life-threatening arrhythmias like VF, the heart’s electrical activity becomes chaotic, causing the muscle to quiver rather than contract effectively, halting blood circulation. A manual defibrillator works by:
- Monitoring: Using adhesive electrodes (pads) or handheld paddles placed on the patient’s chest, the device acquires and displays the patient’s electrocardiogram (ECG) on a screen.
- Analysis: The clinician interprets the ECG rhythm to confirm a shockable rhythm (VF or pulseless VT).
- Energy Selection: The clinician manually selects the appropriate energy level (measured in joules).
- Charging: The device charges its internal capacitors to the selected energy level.
- Delivery: The clinician ensures everyone is clear of the patient and delivers the shock via the pads or paddles. This electrical current depolarizes a critical mass of the heart muscle simultaneously, intending to terminate the chaotic activity and allow the heart’s natural pacemaker to re-establish an effective rhythm.
Key Components
- Main Unit/Base: Houses the electronics, battery, and capacitors.
- Display Screen: Shows the patient’s real-time ECG, heart rate, and often other parameters like oxygen saturation if integrated.
- Energy Selector: A dial or buttons to set the desired shock energy (e.g., 200J, 300J, 360J).
- Charge Button: Initiates the charging of the capacitors.
- Shock Buttons: Physical buttons (often on paddles or on the unit itself) that must be pressed simultaneously to deliver the shock.
- Defibrillation Paddles: Handheld, sterile, metal electrodes for direct chest application. They often have an ECG acquisition function.
- Defibrillation Pads (Adhesive Electrodes): Disposable, adhesive gel pads that are attached to the patient’s chest. They are now standard for most uses as they are safer, provide better contact, and allow for hands-free rhythm monitoring.
- ECG Leads: Ports to connect standard 3-, 5-, or 12-lead ECG cables for more detailed monitoring.
- Synchronization Button (Sync): Used for cardioversion of non-lethal arrhythmias (like atrial fibrillation). It times the shock to deliver on the R-wave of the ECG to avoid causing VF.
- Pacer (optional but common): An integrated transcutaneous pacemaker that can deliver electrical impulses to stimulate the heart externally.
2. Uses
Clinical Applications
- Treatment of Shockable Cardiac Arrest: The primary use is for VF and pulseless VT during resuscitation (CPR).
- Synchronized Cardioversion: For hemodynamically unstable tachyarrhythmias with a pulse, such as unstable atrial fibrillation, atrial flutter, or monomorphic VT. The “sync” mode is used.
- Continuous Cardiac Monitoring: Used in emergency departments, ICUs, and during patient transport for high-fidelity ECG monitoring.
- Transcutaneous Pacing: For symptomatic bradycardias unresponsive to medication, using the integrated pacer function.
Who Uses It
Manual defibrillators are operated by highly trained medical professionals, including:
- Physicians (especially cardiologists, anesthesiologists, emergency doctors, intensivists)
- Registered Nurses (particularly in critical care, emergency, and cardiac units)
- Paramedics and Advanced EMTs in pre-hospital advanced life support systems
- Physician Assistants and Nurse Practitioners
- Cardiac Technologists
Departments/Settings
- Hospital: Emergency Department (ED), Intensive Care Unit (ICU), Cardiac Care Unit (CCU), Operating Room (OR), Post-Anesthesia Care Unit (PACU), Cardiac Catheterization Lab, General Wards (as part of a crash cart).
- Ambulances: Mobile Intensive Care Units (MICUs).
- Outpatient Clinics: Large cardiology or surgical specialty clinics.
3. Technical Specs
Typical Specifications
- Energy Output: Typically ranges from 1-360 Joules (max may be 200J for pediatric).
- Waveform: Biphasic truncated exponential (BTE) is the modern standard; older models used monophasic damped sinusoidal (MDS).
- Battery: Lithium-ion rechargeable, providing 3-5+ hours of operation or 70+ shocks. Has a backup internal battery.
- Display: Color TFT or LED screen, typically 8-12 inches.
- Monitoring: 3/5/12-lead ECG, often with SpO2, NIBP, EtCO2, and temperature capabilities.
- Pacing: Output: 0-200 mA; Rate: 30-180 ppm.
- Weight: 8-15 kg (17-33 lbs) for a portable unit.
Variants & Sizes
- Monitors/Defibrillators: Full-featured units with large screens and multiple parameters (ECG, SpO2, NIBP, Temp, CO2).
- Defibrillator/Monitors: More compact, focused on core defib/pacing/ECG functions.
- Crash Cart Models: Designed to dock into a standardized hospital resuscitation (“crash”) cart.
Materials & Features
- Construction: High-impact plastic housing, often with rubberized bumpers for durability.
- Features:
- Pacing: Demand or fixed-rate transcutaneous pacing.
- 12-Lead ECG: Capability to acquire and print diagnostic-quality 12-lead ECGs.
- Data Recording & Connectivity: Records all ECG and event data (shocks, settings). USB/Bluetooth/Wi-Fi for data transfer to hospital EMR.
- Quick Shock: Allows charging during CPR with minimal pauses.
- Impedance Compensation: Adjusts energy delivery based on patient chest resistance.
- Voice/ECG Prompts: Guides the user through ACLS protocols.
Notable Models
- Philips HeartStart MRx / XL+
- ZOLL R Series / M Series / X Series
- GE Healthcare CARESCAPE R860 / Coro
- Stryker (Physio-Control) LIFEPAK 20e / 15
- Schiller FRED easyport / DEFIGARD Touch 7
4. Benefits & Risks
Advantages
- Clinician Control: Allows for nuanced decision-making based on real-time rhythm interpretation and patient context.
- Multifunctionality: Combines defibrillation, pacing, monitoring, and diagnostics in one device.
- High-Fidelity Data: Provides detailed ECG information crucial for diagnosis and treatment decisions.
- Versatility: Suitable for a wide range of patients, from neonates to adults, with adjustable energy and pad sizes.
Limitations
- Requires Extensive Training: Operator skill is critical for safe and effective use.
- Slower Time to First Shock: Compared to an AED in untrained hands, as analysis is manual.
- Cost: Significantly more expensive than AEDs.
- Size & Weight: Less portable than AEDs, though modern units are quite compact.
Safety Concerns & Warnings
- Operator/Staff Safety: The classic command “Clear!” is paramount. Visually and verbally ensure no one is touching the patient or bed before shocking to prevent accidental electrocution.
- Patient Safety: Incorrect pad placement can reduce effectiveness and cause burns. Avoid placing pads over implantable devices or medication patches.
- Flammable Environments: Oxygen-enriched atmospheres pose a fire risk during arcing. Move oxygen sources away from the chest during shock delivery.
- Device Maintenance: Regular checks are essential to ensure the device is rescue-ready.
Contraindications
- Non-Shockable Rhythms: Do not shock asystole (flatline) or pulseless electrical activity (PEA). It is ineffective and delays correct treatment (CPR/epinephrine).
- Synchronized Mode: Do not use sync mode for VF/VT without a pulse. The device will not shock.
- Conscious, Stable Patient: A conscious patient with a pulse should never receive an unsynchronized shock.
5. Regulation
Manual defibrillators are highly regulated life-critical devices.
- FDA Class: Class III (requires Premarket Approval – PMA due to high risk).
- EU MDR Class: Class III under Rule 9.
- CDSCO Category: Category D (High Risk).
- PMDA (Japan): Classified as “Highly Controlled Medical Devices.”
- ISO/IEC Standards:
- ISO 60601-1: General safety for medical electrical equipment.
- ISO 60601-2-4: Particular requirements for cardiac defibrillators.
- ISO 80601-2-55: Particular requirements for basic safety and essential performance of respiratory gas monitors.
- ISO 13485: Quality management systems for medical devices.
6. Maintenance
Cleaning & Sterilization
- External Unit: Clean with a soft cloth dampened with mild soap and water or a hospital-grade disinfectant (e.g., 70% isopropyl alcohol). Never immerse the unit. Avoid harsh chemicals.
- Paddles: Clean with alcohol wipes. Some have disposable, sterile paddle covers for single use.
- Cables & Leads: Wipe down according to manufacturer instructions.
Reprocessing
Disposable pads are single-patient use. Paddles, if reused without covers, require low-level disinfection between patients.
Calibration
Devices perform daily/weekly/monthly self-tests automatically. Formal performance verification and calibration (checking energy output accuracy, pacing current, etc.) should be performed annually or as per manufacturer/hospital protocol by a certified biomedical engineer.
Storage
- Store in a dry, clean environment on a dedicated crash cart or wall mount.
- Temperature: Typically 0°C to 50°C (32°F to 122°F).
- Keep the main and backup batteries fully charged. Most units have a “plugged-in” mode for storage.
- Ensure spare sets of adult/pediatric pads are available and within expiry date.
7. Procurement Guide
How to Select the Device
Consider: Clinical need (ED vs. ward transport), budget, ease of use, durability, battery life, connectivity, and service support.
Quality Factors
- Reliability & Uptime: Proven track record of performance in emergencies.
- Screen Visibility: Bright, clear display readable in all lighting.
- Battery Performance: Long life and short recharge time.
- Durability: Ruggedized design to withstand bumps and drops.
- Intuitive Interface: Minimizes confusion during high-stress situations.
Certifications
Look for regulatory marks: FDA Clearance, CE Mark, ISO 13485 certification. Country-specific marks (e.g., JPAL for Japan) are required for local markets.
Compatibility
- Pads/Electrodes: Must be compatible with the specific defibrillator model.
- Hospital Network: Compatibility with central monitoring stations and EMR systems via HL7/ICE protocols is crucial.
- Crash Carts: Docking compatibility with existing hospital crash carts.
Typical Pricing Range
Manual defibrillators are capital equipment. Prices vary widely based on features.
- Basic Defib/Monitor/Pacer: \$8,000 – \$15,000
- Full-Featured Monitor/Defibrillator (with 12-lead, advanced parameters): \$15,000 – \$30,000+
8. Top 10 Manufacturers (Worldwide)
- Philips Healthcare (Netherlands/USA): A global leader. Notable Line: HeartStart MRx, IntelliVue X.
- ZOLL Medical (USA, a subsidiary of Asahi Kasei): Specializes in resuscitation. Notable Line: R Series, X Series.
- GE Healthcare (USA): Major player in medical imaging and monitoring. Notable Line: CARESCAPE R860, Coro.
- Stryker (USA) – Physio-Control: Historically synonymous with defibrillators (LIFEPAK). Notable Line: LIFEPAK 20e, 15.
- Mindray (China): Rapidly growing global provider. Notable Line: BeneHeart D Series, iMEC series.
- Schiller AG (Switzerland): Strong in cardiology diagnostics and emergency care. Notable Line: DEFIGARD Touch 7, FRED easyport.
- Nihon Kohden (Japan): Leading Japanese manufacturer of medical electronic equipment. Notable Line: TEC-5600 series.
- BPL Medical Technologies (India): Leading Indian manufacturer. Notable Line: Dual系列.
- Bexen Cardio (Spain): Specializes in emergency and critical care equipment.
- Metrax GmbH (Germany) – PRIMEDIC: Manufacturer of defibrillators and emergency medical products.
9. Top 10 Exporting Countries (Latest Year – Based on HS Code 9018.90* trends)
Note: Specific annual ranking fluctuates. The following is indicative of major exporting nations.
- United States: Home to Philips, ZOLL, GE, Stryker. The largest hub for high-tech device innovation and export.
- Germany: A traditional powerhouse in medical engineering (e.g., Metrax/PRIMEDIC, Schiller has a strong presence).
- Netherlands: Philips’ operational HQ drives significant exports.
- China: Massive manufacturing base, with Mindray as a global exporter.
- Japan: Home to Nihon Kohden, a key supplier in Asia and globally.
- Switzerland: Precision engineering hub (Schiller AG).
- Ireland: Many US manufacturers have EU distribution centers here.
- Singapore: A major regional distribution hub for Asia-Pacific.
- United Kingdom: Houses European HQs of several major players.
- France: Strong domestic and export market for medical devices.
10. Market Trends
Current Global Trends
- Integration & Connectivity: Seamless data flow to EMR and cloud-based analytics for post-event review and quality improvement.
- Portability & Ruggedization: Demand for lighter, more durable units for use across diverse hospital and pre-hospital settings.
- Guidance-Driven Resuscitation: Devices increasingly provide real-time CPR feedback (rate, depth, recoil) and ACLS algorithm prompts.
New Technologies
- AI-Assisted Rhythm Analysis: Algorithms to support (not replace) clinician interpretation, reducing analysis time.
- Multi-Parameter Monitoring: Integration of advanced hemodynamic monitoring (e.g., invasive BP, cardiac output).
- Longer-Life Batteries & Fast Charging.
Demand Drivers
- Rising global incidence of cardiovascular diseases.
- Aging populations.
- Stringent regulations mandating advanced resuscitation equipment in more healthcare settings.
- Hospital focus on improving “Code Blue” survival rates.
Future Insights
The manual defibrillator will remain the gold-standard for in-hospital resuscitation. Its future lies in becoming the intelligent central hub of the resuscitation ecosystem—guiding the entire team with real-time data, integrating point-of-care ultrasound (POCUS), and automating documentation to allow clinicians to focus purely on patient care.
11. Training
Required Competency
Competency is non-negotiable. Requires:
- Certification in Advanced Cardiac Life Support (ACLS).
- Manufacturer-specific device training.
- Regular, hands-on simulation drills (at least biannually) covering rhythm recognition, energy selection, synchronized cardioversion, and pacing.
Common User Errors
- Failure to “Clear” Effectively: Leading to team member injury.
- Incorrect Pad Placement: Reduces shock efficacy.
- Shocking Non-Shockable Rhythms: Wasting time during arrest.
- Forgetting to Engage “Sync” Mode for cardioversion of a tachyarrhythmia with a pulse (risks inducing VF).
- Poor CPR Integration: Pausing CPR for too long before/after shock delivery.
Best-Practice Tips
- “Pads Over Paddles”: Use adhesive pads for safety, continuous monitoring, and hands-free operation.
- Charging During CPR: Charge the device during ongoing chest compressions to minimize pre-shock pause.
- Follow the Screen: Use the on-screen ECG for rhythm analysis, not just the defibrillator paddles.
- Post-Shock: Immediately resume CPR for 2 minutes without rechecking the pulse/rhythm, as per ACLS guidelines.
12. FAQs
Q1: What’s the main difference between a manual defibrillator and an AED?
A: An AED automatically analyzes the rhythm and tells the user if a shock is advised. A manual defibrillator displays the ECG for a trained professional to interpret and make the shock decision manually. Manual units also have pacing and advanced monitoring.
Q2: Can you use a manual defibrillator on a pediatric patient?
A: Yes, but you must use pediatric-specific pads or a dose attenuator (if available on the device) to reduce the energy output. The energy dose is weight-based (e.g., 2-4 J/kg).
Q3: Why do we say “Clear!” and look around before shocking?
A: To ensure no one is in direct or indirect contact with the patient. The electrical current can arc and travel, causing a serious shock to anyone touching the patient or even the bed.
Q4: What happens if you shock a patient in asystole (flatline)?
A: Nothing beneficial. It will not restart the heart. It wastes critical time during a resuscitation where the correct treatments are high-quality CPR and epinephrine.
Q5: What is “synchronized” cardioversion?
A: It’s for shocking a patient with a pulse but an unstable, fast rhythm (like atrial fibrillation). The device times the shock to deliver on the R-wave of the ECG to avoid triggering VF.
Q6: How often should the defibrillator be checked?
A: It should be inspected visually at the start of every shift (pads, battery, cables). A full function test (including a discharge test into a test load) is performed daily/weekly by clinical staff and annually by biomedical engineering.
Q7: Can you defibrillate a patient who is on a wet surface or has a nitroglycerin patch on?
A: Dry the chest if wet to prevent current dispersion. Always remove any medication patches from the chest area and wipe the skin clean before applying pads to prevent burns and arcing.
Q8: What does a “biphasic waveform” mean?
A: It means the electrical current flows in one direction for a set duration, then reverses and flows back. This is more efficient than old monophasic shocks, allowing for lower energy doses (e.g., 200J vs. 360J) to be effective, causing less potential myocardial damage.
13. Conclusion
The manual defibrillator is a cornerstone of modern emergency and critical care medicine. Its power lies not just in its ability to deliver a life-saving shock, but in the sophisticated diagnostic and therapeutic control it places in the hands of a trained clinician. From the chaos of a cardiac arrest to the controlled procedure of a cardioversion, it is an essential tool. Its effective use hinges on a triad of factors: a reliable, well-maintained device, comprehensive, ongoing training, and adherence to standardized protocols. As technology evolves, integrating smarter guidance and connectivity, the fundamental role of the skilled operator interpreting the rhythm and leading the team remains paramount. Understanding this device—its capabilities, limitations, and proper operation—is fundamental for any healthcare provider in a position to respond to a cardiac emergency.
14. References
- American Heart Association. (2020). Advanced Cardiac Life Support (ACLS) Provider Manual.
- International Organization for Standardization. ISO 60601-2-4:2019 – Medical electrical equipment — Part 2-4: Particular requirements for the basic safety and essential performance of cardiac defibrillators.
- U.S. Food and Drug Administration. (2023). Classify Your Medical Device.
- European Commission. (2017). Regulation (EU) 2017/745 on medical devices (MDR).
- World Health Organization. (2020). Technical specifications for automated external defibrillators.
- Manufacturer Operator Manuals: Philips HeartStart MRx, ZOLL R Series, GE CARESCAPE R860.
- Journal of Emergency Medical Services (JEMS). (2023). The Future of Resuscitation Technology.
- Resuscitation (Journal of the European Resuscitation Council).