1. Definition
What is an ICU Ventilator?
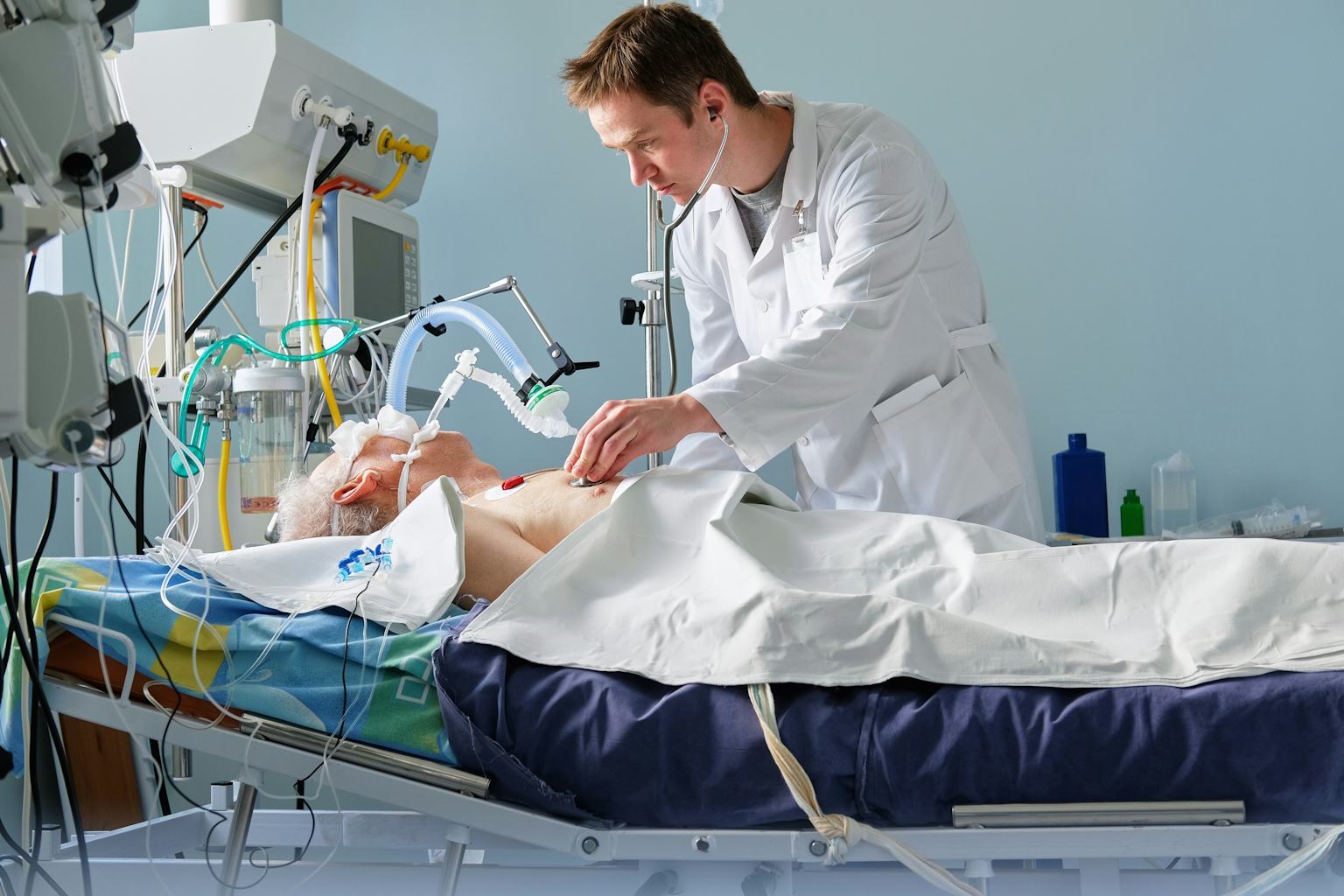
An ICU (Intensive Care Unit) ventilator, often called a mechanical ventilator, respirator, or life-support machine, is a critical care medical device designed to assist or replace spontaneous breathing in patients who are unable to breathe adequately on their own. It is a complex, microprocessor-controlled system that delivers precisely measured volumes or pressures of a gas mixture (typically oxygen-enriched air) into a patient’s lungs through an artificial airway, such as an endotracheal or tracheostomy tube. Its primary function is to maintain adequate oxygenation and carbon dioxide removal (ventilation) for patients with respiratory failure, allowing the body to heal.
How it Works
At its core, a ventilator works by applying positive pressure to push breathable gas into the lungs—a process called inspiration. It then allows passive exhalation by releasing that pressure. The ventilator’s computer (control system) orchestrates this cycle based on preset parameters entered by the clinician. Here’s the simplified cycle:
- Trigger: The ventilator detects the patient’s own attempt to breathe (patient-triggered) or follows a set timing (machine-triggered).
- Inspiration: The machine’s internal compressor or gas source delivers the breath, controlling either the volume (how much air) or the pressure (the force with which it’s delivered).
- Cycle: The ventilator switches from inspiration to exhalation based on a set volume, time, or flow threshold.
- Expiration: The machine’s exhalation valve opens, letting the patient exhale passively due to the natural elasticity of the lungs. Positive End-Expiratory Pressure (PEEP) is often applied to prevent the lungs from fully collapsing at the end of exhalation.
Key Components
- Control System/User Interface: The computer brain and touchscreen/knob interface for setting parameters and monitoring data.
- Drive Mechanism: The motor (turbine or compressor) that generates the gas flow.
- Gas Blending System: Mixes medical air and oxygen to achieve the desired fraction of inspired oxygen (FiO₂).
- Breathing Circuit: A set of disposable tubes connecting the ventilator to the patient’s airway. It includes an inspiratory limb and an expiratory limb.
- Valves: Precise valves control the direction, flow, and pressure of gas (inspiratory, exhalation, and PEEP valves).
- Humidifier: Warms and humidifies the dry medical gas to protect the patient’s lung tissue.
- Sensors: Measure pressure, flow, and volume at various points to provide real-time feedback to the control system for safe operation.
- Alarms: Audible and visual alerts for issues like high/low pressure, disconnection, apnea, or gas supply failure.
- Battery: Provides backup power during transport or power failure.
2. Uses
Clinical Applications
ICU ventilators are used for a wide spectrum of conditions causing respiratory failure:
- Acute Respiratory Distress Syndrome (ARDS): Provides lung-protective ventilation strategies with low tidal volumes and precise PEEP management.
- Post-operative Care: Supports breathing after major surgeries, especially cardiac or thoracic procedures.
- Neuromuscular Diseases: Replaces breathing effort in conditions like Guillain-Barré syndrome, myasthenia gravis, or spinal cord injury.
- Drug Overdose/Anesthesia: Manages respiration depressed by sedatives, opioids, or general anesthetics.
- Severe Pneumonia/COVID-19: Supports gas exchange while the infection is treated.
- Trauma: Manages respiratory failure from chest injuries or traumatic brain injury.
- Chronic Obstructive Pulmonary Disease (COPD) Exacerbation: Assists fatigued respiratory muscles.
- Sepsis/Shock: Manages the acute respiratory failure that often accompanies severe systemic infection.
Who Uses It
- Intensivists/Critical Care Physicians: Prescribe the ventilation strategy and key parameters.
- Respiratory Therapists: Are the primary experts in setting up, operating, monitoring, and adjusting the ventilator based on blood gas analysis and patient response.
- Critical Care Nurses: Monitor the patient-ventilator interface, manage patient comfort (sedation, positioning), and respond to initial alarms.
- Anesthesiologists: Use ventilators in the operating room and during patient transport to the ICU.
Departments/Settings
Primarily used in Intensive Care Units (Medical, Surgical, Cardiac, Neurological, Pediatric, Neonatal). Also found in Operating Rooms (OR), Post-Anesthesia Care Units (PACU), and during critical care patient transport (via specialized transport ventilators).
3. Technical Specs
Typical Specifications
- Tidal Volume: Range from 2 mL to 2500 mL (covers neonates to large adults).
- Respiratory Rate: 1 to 150 breaths per minute.
- Pressure Support: 0 to 80 cm H₂O.
- PEEP Range: 0 to 45 cm H₂O.
- FiO₂ Range: 21% to 100%.
- Peak Inspiratory Pressure Limit: Up to 120 cm H₂O.
- Monitoring: Displays waveforms (pressure, flow, volume), loops (Pressure-Volume, Flow-Volume), compliance, resistance, and minute ventilation.
- Power Supply: AC Mains with internal battery (2-8 hours backup).
Variants & Sizes
- ICU Ventilators: Full-featured, stationary workhorses for the ICU.
- Transport Ventilators: Rugged, battery-powered, with essential modes for moving patients.
- Neonatal/Pediatric Ventilators: Specialized for the unique physiology and small volumes of infants and children, often integrated into ICU ventilators as a software/hardware package.
Materials & Features
- Construction: Durable medical-grade plastics and metals for the main unit; single-use, biocompatible plastics for circuits.
- Advanced Features: Integrated touchscreens, lung mechanics monitoring, non-invasive ventilation (NIV) modes, advanced modes (APRV, ASV, NAVA), built-in suction, and connectivity for EMR (Electronic Medical Record) integration.
- Innovations: Automated weaning protocols, closed-loop control modes, tele-ventilation capabilities, and advanced nebulizer integration.
Notable Models
- Hamilton Medical (Switzerland): Hamilton-G5, Hamilton-C6 (featuring Adaptive Support Ventilation – ASV).
- Getinge (Sweden): Servo-u, Servo-n (for neonatal care).
- Dräger (Germany): Evita V800, Savina 300.
- Medtronic (Ireland/USA): Puritan Bennett 980 (PB980).
- Philips Respironics (Netherlands/USA): V60 (for NIV/Transport), Trilogy Evo.
- Vyaire Medical (USA): AVEA, VELA.
- GE Healthcare (USA): CARESCAPE R860.
- Lowenstein Medical (Germany): ELISA 800.
- Mindray (China): SV800, SV70.
- Maquet (Getinge) (Sweden): Servo-i.
4. Benefits & Risks
Advantages
- Life-Sustaining: The primary benefit is supporting or replacing failing lung function, buying time for treatment and recovery.
- Improves Oxygenation & Ventilation: Prevents hypoxia (low oxygen) and manages hypercapnia (high CO₂).
- Reduces Work of Breathing: Allows respiratory muscles to rest, preventing fatigue.
- Precise Control: Enables delivery of lung-protective strategies, reducing Ventilator-Induced Lung Injury (VILI).
- Comprehensive Monitoring: Provides continuous data on patient lung mechanics and response to therapy.
Limitations
- Invasiveness: Requires sedation and an artificial airway, which carries its own risks (infection, injury).
- Complexity: Requires extensive training to operate safely and effectively.
- Cost: High capital and ongoing costs (disposables, maintenance).
- Patient Discomfort: Can cause anxiety, requiring sedation and sometimes paralysis.
- Immobility: Can confine the patient to bed, contributing to muscle weakness.
Safety Concerns & Warnings
- Ventilator-Associated Pneumonia (VAP): A major risk due to the bypassing of natural airway defenses.
- Barotrauma/Volutrauma: Lung injury from excessive pressure or volume (e.g., pneumothorax).
- Atelectrauma: Injury from repeated opening and collapsing of alveoli.
- Disconnection or Leak: Can lead to rapid hypoxia.
- Alarm Fatigue: The high frequency of alarms can lead to desensitization.
- Power/Gas Supply Failure: Requires robust backup systems.
Contraindications
There are no absolute contraindications to life-saving ventilation. However, certain situations require extreme caution or special consideration:
- Untreated Tension Pneumothorax: Must be decompressed before positive pressure ventilation.
- Severe Facial Trauma/Burns: May complicate mask fitting for non-invasive ventilation.
- Recent Airway Surgery: Requires specialized consultation.
- Patients with a “Do Not Intubate” (DNI) order: Limits the use to non-invasive methods only.
5. Regulation
ICU ventilators are high-risk devices and are strictly regulated worldwide.
- FDA Class: Class II (Special Controls) for most critical care ventilators (product code BZD). Some with advanced functionality may be Class III.
- EU MDR Class: Class IIb (for devices intended for respiratory support lasting more than 24 hours).
- CDSCO Category (India): Class C (Moderate to High Risk), equivalent to a risk level where manufacturing license is required from the Central Licensing Authority.
- PMDA Notes (Japan): Classified as “Highly Controlled Medical Devices.” Requires pre-market approval (PMA) with rigorous clinical data submission.
- ISO/IEC Standards:
- ISO 80601-2-12: The fundamental safety and performance standard for critical care ventilators.
- ISO 80601-2-80: For ventilatory support equipment for the vulnerable patient population (neonatal).
- IEC 60601-1: General standard for the safety of medical electrical equipment.
6. Maintenance
Cleaning & Sterilization
- External Surfaces: Wipe down daily and after each patient use with hospital-grade disinfectant wipes.
- Internal Components: Sealed and not user-serviceable. Air pathways are protected by bacterial/viral filters.
- Breathing Circuits & Humidifiers: Single-use only. Discarded after each patient. Never reprocess.
Reprocessing
ICU ventilators themselves are not reprocessed between patients. The patient circuit (tubes, filters, humidifier chamber), which is the only part contacting patient gases, is disposed of. The machine’s internal gas pathway is protected by an external filter. A full preventive maintenance check is performed between patients.
Calibration
Performed periodically (e.g., annually or per manufacturer specs) by certified biomedical engineers. This includes verifying the accuracy of pressure, flow, and oxygen sensors. Modern ventilators often have extensive self-test and calibration routines built-in.
Storage
Store in a clean, dry, temperature-controlled environment. When not in use for prolonged periods, follow the manufacturer’s “mothballing” procedure, which may include running the device periodically to keep internal components functional. Ensure backup batteries are charged on a schedule.
7. Procurement Guide
How to Select the Device
Consider:
- Patient Population: Adult, pediatric, neonatal, or all-in-one?
- Clinical Needs: Required ventilation modes (standard, advanced, NIV), monitoring capabilities.
- Ease of Use: Intuitive interface, quick-setup features, alarm management.
- Durability & Uptime: Reliability track record, mean time between failures.
- Service & Support: Local technical support, service contract terms, parts availability.
- Connectivity: Compatibility with hospital monitoring networks and EMR.
Quality Factors
- Performance Consistency: Accurate delivery of set parameters under varying lung conditions.
- Alarm Reliability: Low false alarm rate with high sensitivity for true events.
- Graphical Interface: Clear, customizable waveforms and trends.
- Battery Life: Sufficient for intra-hospital transport or power outages.
Certifications
Look for CE Marking (for EU), FDA 510(k) Clearance or PMA (for USA), and compliance with ISO 80601-2-12. Other regional certifications like INMETRO (Brazil), TGA (Australia) may be relevant.
Compatibility
Ensure compatibility with existing hospital gas outlets (air/O2 pressures), electrical systems, patient monitors, and EMR interfaces. Check if it can use standard or proprietary breathing circuits.
Typical Pricing Range
A full-featured ICU ventilator typically ranges from $25,000 to $50,000 USD. Final cost depends on configuration, service packages, and volume. Transport ventilators range from $10,000 to $20,000.
8. Top 10 Manufacturers (Worldwide)
- Hamilton Medical AG (Switzerland): A global leader known for its intelligent ventilation modes like ASV. Flagship: Hamilton-G5.
- Getinge AB (Sweden): A giant in medical technology, its Maquet division produces the renowned Servo series. Flagship: Servo-u.
- Drägerwerk AG & Co. KGaA (Germany): A historic leader in gas technology and medical devices with a strong ICU portfolio. Flagship: Evita V800.
- Medtronic plc (Ireland/USA): Acquired Covidien, inheriting the long-standing Puritan Bennett brand. Flagship: PB980.
- Philips Respironics (Netherlands/USA): Strong in sleep and respiratory care, with a significant presence in NIV and transport. Flagship: V60, Trilogy.
- Vyaire Medical, Inc. (USA): Spun off from BD, focused solely on respiratory care with products like AVEA and VELA.
- GE HealthCare (USA): Offers the CARESCAPE R860 as part of its integrated critical care ecosystem.
- Mindray Bio-Medical Electronics Co., Ltd. (China): A rapidly growing global player offering high-spec devices at competitive prices. Flagship: SV800.
- Löwenstein Medical Technology GmbH & Co. KG (Germany): Known for innovation in NIV and critical care ventilation. Flagship: ELISA 800.
- Fisher & Paykel Healthcare Ltd. (New Zealand): A world leader in humidification, also producing dedicated ICU ventilators like the AIRVO 2 (for oxygen therapy) and critical care systems.
9. Top 10 Exporting Countries (Latest Year – Based on 2022/2023 Trend Data)
(Note: Exact ranking fluctuates annually; this reflects major production hubs.)
- Germany: A traditional engineering powerhouse, home to Dräger and Getinge (Maquet) production. High-value, precision exports.
- United States: Major hub for Medtronic, Vyaire, and Philips production for the Americas market.
- Switzerland: Home to Hamilton Medical, a key exporter of high-end devices.
- China: A massive and growing manufacturing base, led by Mindray, exporting globally across all price segments.
- Ireland: A significant Medtronic manufacturing site for exports to EMEA and beyond.
- Singapore: A regional manufacturing hub for many multinationals (e.g., Siemens Healthineers historically) serving Asia-Pacific.
- Sweden: Home to Getinge’s headquarters and production.
- Netherlands: Philips’ Respironics division exports from here.
- Mexico: An important manufacturing location for the North American market, particularly for US-based companies.
- Italy: Hosts production facilities for several European manufacturers.
10. Market Trends
Current Global Trends
- Post-Pandemic Capacity Building: Hospitals worldwide are bolstering ventilator inventories and upgrading to more versatile units.
- Rising Chronic Respiratory Diseases: Increasing prevalence of COPD and sleep apnea is driving demand for versatile devices that can handle both invasive and non-invasive support.
- Cost-Conscious Procurement: In many markets, there is a growing acceptance of high-quality devices from emerging manufacturers (e.g., Mindray) offering competitive pricing.
New Technologies
- Closed-Loop & Automated Weaning: Systems like INTELLiVENT-ASV automatically adjust settings based on patient needs, aiming to reduce ventilator time and clinician workload.
- Tele-Ventilation: Remote monitoring of ventilator data, allowing off-site specialists to support bedside teams.
- Advanced Monitoring Integration: Ventilators acting as data hubs, integrating CO₂, EEG, or hemodynamic data to guide therapy (e.g., for ARDS or brain-injured patients).
- Improved HMI & Decision Support: Touchscreen interfaces with configurable views and smart alarms that suggest parameter adjustments.
Demand Drivers
- Aging global population.
- Increasing number of ICUs and critical care beds in developing economies.
- Rising incidence of pandemics/respiratory outbreaks.
- Technological advancements encouraging upgrades.
- Focus on reducing Ventilator-Associated Events (VAEs).
Future Insights
The future points towards smart, integrated, and minimally invasive ventilation. Ventilators will become more adaptive, using AI to personalize therapy and predict weaning readiness. There will be a stronger push for modes that promote spontaneous breathing and patient-ventilator synchrony to reduce sedation and ICU-acquired weakness. Connectivity will be standard, feeding into hospital-wide patient data analytics platforms.
11. Training
Required Competency
Operators must be formally trained and credentialed. This typically involves:
- Understanding pulmonary physiology and pathophysiology of respiratory failure.
- Knowledge of all ventilator modes, parameters, and their clinical implications.
- Proficiency in interpreting waveforms, loops, and numerical data.
- Ability to perform routine checks, troubleshoot alarms, and respond to emergencies.
- Training is mandatory for Respiratory Therapists and Critical Care Nurses, and essential for Critical Care Physicians.
Common User Errors
- Incorrect Trigger Sensitivity Setting: Leading to auto-triggering or failure to trigger, causing patient-ventilator asynchrony.
- Inappropriate PEEP Setting: Too low (risking atelectasis) or too high (risking barotrauma/cardiac depression).
- Ignoring or Silencing Alarms: Instead of identifying and addressing the root cause.
- Failure to Check/Change Filters: Leading to increased work of breathing or device malfunction.
- Misinterpreting Pressure Readings: Confusing peak pressure with plateau pressure.
Best-Practice Tips
- Start with Lung-Protective Settings: Especially for ARDS (low tidal volume 4-8 mL/kg, adequate PEEP).
- Assess Synchrony Regularly: Watch the patient and the waveforms; adjust settings to improve comfort.
- Perform Daily Spontaneous Breathing Trials (SBTs): The key to timely liberation from the ventilator.
- Maintain Circuit Integrity: Ensure connections are tight, circuits are free of water, and the heat-moisture exchanger is changed as needed.
- Know Your Emergency Procedures: Manual ventilation (Ambu bag) must be immediately available. Practice power/gas failure drills.
12. FAQs
1. What’s the difference between an ICU ventilator and an anesthesia machine ventilator?
Anesthesia ventilators are designed for short-term, controlled ventilation in sedated patients during surgery, often with simpler modes. ICU ventilators are built for long-term support of critically ill patients with varying lung pathology, offering a wider range of sophisticated modes, extensive monitoring, and alarms for autonomous operation.
2. Can a ventilator cure a lung disease?
No. A ventilator does not cure the underlying condition. It is a supportive therapy that manages breathing while the disease is treated with medications, procedures, and time, allowing the lungs to heal.
3. How long can a patient be on a ventilator?
It varies from hours (post-surgery) to weeks or even months (severe ARDS, neuromuscular disease). Prolonged ventilation increases the risk of complications like VAP, muscle weakness, and delirium.
4. Is being on a ventilator painful?
The endotracheal tube can be uncomfortable, and the sensation of being unable to breathe naturally can cause anxiety. Therefore, patients are usually given sedatives and analgesics for comfort.
5. What does “weaning” from a ventilator mean?
Weaning is the gradual process of reducing ventilator support as the patient’s own breathing ability improves, culminating in a trial of breathing without assistance (Spontaneous Breathing Trial) and, if successful, removal of the breathing tube (extubation).
6. Why are alarms constantly going off?
Common reasons: patient coughing or biting the tube, secretions in the airway, condensation (“rain-out”) in the circuit, leaks, or the patient’s breathing effort changing. It requires assessment, not just silencing.
7. What is PEEP and why is it important?
PEEP (Positive End-Expiratory Pressure) keeps a small amount of pressure in the lungs at the end of exhalation. This prevents alveoli from collapsing, improves oxygenation, and can reduce lung injury.
8. What happens if the power goes out?
ICU ventilators have internal batteries that automatically engage, typically providing 2-8 hours of power. Hospitals also have backup generators that should start within seconds.
9. Can a patient talk or eat while on a ventilator?
Not with a standard endotracheal tube through the mouth/nose. If ventilation is required long-term, a tracheostomy tube may be placed. With a special talking tracheostomy tube or valve, some speech may be possible. Eating is generally not possible; nutrition is provided via a feeding tube.
10. What is the survival rate for patients on ventilators?
This varies tremendously based on the underlying reason for ventilation. For some conditions (e.g., post-surgery), it is very high. For others (e.g., severe ARDS or multi-organ failure), mortality can be significant (30-50% or higher). The prognosis is specific to the individual patient’s illness.
13. Conclusion
The ICU ventilator is a pinnacle of modern life-support technology, a complex yet indispensable tool in the fight against respiratory failure. Its effective use requires a deep synthesis of technology and clinical art—understanding not just the knobs and screens, but the pathophysiology of the patient’s lungs. From stringent global regulations and sophisticated maintenance protocols to the continuous evolution driven by smart technology and market needs, the ventilator ecosystem is dynamic. Success hinges on selecting the right device, ensuring rigorous training for the clinical team, and adhering to best practices focused on lung protection and timely weaning. Ultimately, it is a bridge to recovery, providing vital support while the body’s innate healing capacities take over.
14. References
- International Organization for Standardization. (2019). ISO 80601-2-12: Medical electrical equipment — Part 2-12: Particular requirements for basic safety and essential performance of critical care ventilators.
- U.S. Food and Drug Administration. (2022). Ventilators and Ventilator Accessories EUAs. Retrieved from https://www.fda.gov
- Tobin, M. J. (Ed.). (2012). Principles and Practice of Mechanical Ventilation (3rd ed.). McGraw-Hill Education.
- GlobalData Healthcare. (2023). Mechanical Ventilators Market Size and Share Analysis.
- Chathurn, R. L., & Mireles-Cabodevila, E. (2022). Handbook of Mechanical Ventilation. Elsevier.
- European Centre for Disease Prevention and Control. (2017). Healthcare-associated infections: surveillance and prevention of ventilator-associated pneumonia.
- The Lancet Respiratory Medicine. (2023). The future of mechanical ventilation: perspectives on the next 50 years.
- Manufacturer Websites & Technical Manuals: Hamilton Medical, Getinge, Dräger, Medtronic, Philips.