1. Definition
What is a CPAP Device?
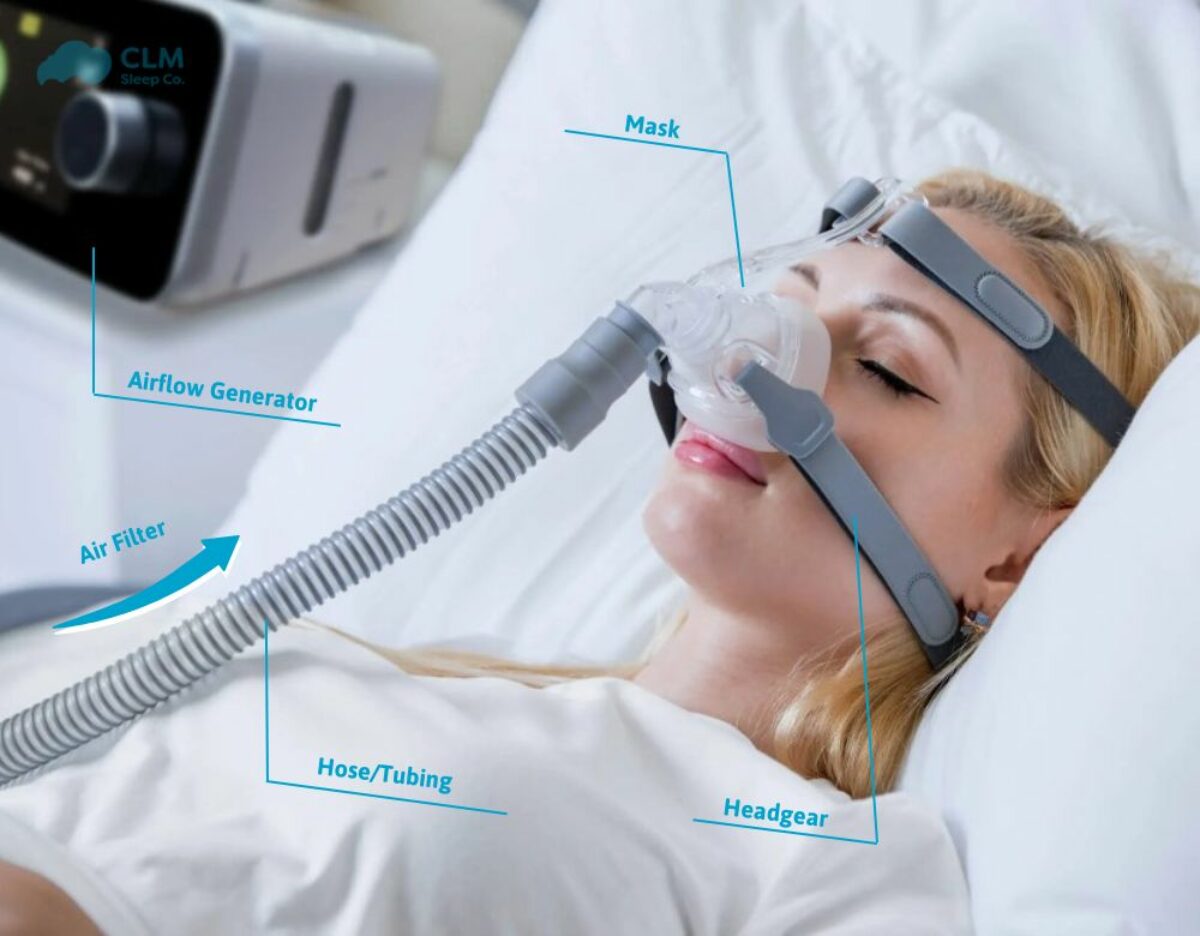
A Continuous Positive Airway Pressure (CPAP) device is a medical-grade respiratory support system designed primarily to treat Obstructive Sleep Apnea (OSA) and other breathing-related sleep disorders. At its core, it is a non-invasive ventilator that prevents the collapse of a patient’s airway during sleep by delivering a constant, prescribed stream of pressurized air through a mask. Think of it as a pneumatic splint that keeps your breathing passage open, allowing for uninterrupted oxygen flow and restful sleep. Beyond sleep apnea, modern CPAP devices are versatile tools used in various clinical settings to manage respiratory distress.
How it Works
The working principle of a CPAP device is elegantly simple yet profoundly effective. It consists of a small, quiet motor (blower unit) that draws in room air. This air is filtered, pressurized to a clinician-prescribed level (measured in centimeters of water pressure, cm H₂O), and then humidified (in most models) for comfort. The pressurized air is delivered via a flexible tube (air circuit) to a mask that seals over the patient’s nose, mouth, or both. This constant gentle pressure acts as a physical barrier, preventing the soft tissues in the throat (like the tongue and uvula) from collapsing and obstructing the airway when the throat muscles relax during sleep. It is a continuous pressure, meaning it remains the same during both inhalation and exhalation, though many devices now feature pressure relief technology to make exhalation feel more natural.
Key Components
- Main Unit/Blower: The core device housing the motor, control electronics, and processor. It generates and regulates the airflow pressure.
- Motor: A precisely controlled, quiet motor that creates the airflow and pressure.
- Air Filter: A disposable or reusable filter that cleans incoming room air of dust and allergens.
- Humidifier (Integrated or Attached): A water chamber that heats and moisturizes the air to prevent nasal, mouth, and throat dryness. It can be heated (for enhanced humidity) or pass-over (cool).
- Air Tubing: A lightweight, flexible hose (typically 6 feet long) that delivers air from the device to the mask. Some are standard, while others are heated to prevent condensation (“rainout”).
- Mask System: The patient interface. Several types exist:
- Nasal Pillow Mask: Small cushions that seal at the nostrils.
- Nasal Mask: Covers the nose only.
- Full Face Mask: Covers both the nose and mouth.
- Hybrid Masks: A combination of styles.
- Headgear: Adjustable straps that keep the mask securely and comfortably in place.
- Power Supply & Cord: Converts AC power to DC for the device. Many have backup battery capabilities.
2. Uses
Clinical Applications
- Obstructive Sleep Apnea (OSA): The primary and most common application. CPAP is the gold-standard treatment for moderate to severe OSA.
- Hypoxemia: Used to improve oxygen levels in patients with various conditions causing low blood oxygen.
- Cardiac-Related Pulmonary Edema: Can help improve oxygenation in conditions like congestive heart failure.
- Neonatal Care: Specialized CPAP devices are used in NICUs to support premature infants with underdeveloped lungs, preventing airway collapse.
- Post-Operative Care: May be used to support patients recovering from anesthesia or surgery who are at risk of respiratory depression.
- Pandemic/Viral Respiratory Support: Used as a supportive measure for patients with respiratory distress from illnesses like COVID-19, often as a step before intubation and mechanical ventilation.
Who Uses It
- Patients: For nightly, at-home therapy.
- Sleep Technicians & Respiratory Therapists: They conduct sleep studies (polysomnography), titrate pressure settings, and fit masks.
- Pulmonologists & Sleep Medicine Physicians: Diagnose conditions, prescribe CPAP therapy, and monitor patient progress.
- Home Healthcare Providers & DME (Durable Medical Equipment) Specialists: Supply, set up, and provide patient education on home devices.
- Critical Care Nurses & Physicians: In hospital settings (ICU, ER) for acute respiratory management.
Departments/Settings
- Sleep Laboratories/Clinics
- Pulmonology and Sleep Medicine Departments
- Home Healthcare Settings
- Intensive Care Units (ICU) & Emergency Rooms
- Neonatal Intensive Care Units (NICU)
- Post-Anesthesia Care Units (PACU)
3. Technical Specs
Typical Specifications
- Pressure Range: Typically 4 to 20 cm H₂O (or 25 cm H₂O for some models).
- Ramp Feature: Allows pressure to start lower and gradually increase to the prescribed level over 5-45 minutes to aid falling asleep.
- Data Recording: Standard on most devices—records usage hours, Apnea-Hypopnea Index (AHI), leak rate, and event flags.
- Connectivity: Modern devices feature Bluetooth, Wi-Fi, or cellular modems for remote patient monitoring.
- Sound Level: Usually below 30 decibels (quieter than a whisper).
- Dimensions: Approx. 6 x 4 x 3 inches for travel units; larger for standard bedside units with integrated humidifiers.
- Weight: 1 to 3 pounds for the main unit.
Variants & Sizes
- Fixed-Pressure CPAP: Delivers one constant pressure setting. Simple and reliable.
- Auto-CPAP (APAP): Automatically adjusts pressure throughout the night based on detected breathing events, snoring, or flow limitation. Provides the minimum pressure needed.
- Bi-Level PAP (BPAP): Provides two distinct pressures: a higher one for inhalation (IPAP) and a lower one for exhalation (EPAP). Used for more complex conditions like Central Sleep Apnea or COPD.
- Travel CPAP: Compact, lightweight, often battery-operated, sometimes without a humidifier.
Materials & Features
- Materials: High-grade, medical-safe plastics (ABS, polycarbonate), silicone for masks and seals, aluminum for heat plates in humidifiers.
- Key Features:
- Expiratory Pressure Relief (EPR/CFlex/A-Flex): Momentarily lowers pressure at the start of exhalation for comfort.
- Heated Humidification with Climate Control: Automatically adjusts hose air temperature to prevent condensation.
- Mask Fit Check: A feature that tests for air leaks once the mask is on.
- Altitude Compensation: Automatically adjusts motor output for use at high altitudes.
Notable Models (Examples)
- ResMed AirSense 10/11 CPAP & AutoSet
- Philips Respironics DreamStation 2 (Note: Subject to past recalls; verify status)
- DeVilbiss IntelliPAP 2
- Fisher & Paykel SleepStyle
- 3B Medical Luna G3
4. Benefits & Risks
Advantages
- Effectively Eliminates Apneas/Hypopneas: Dramatically reduces or stops breathing events, restoring normal sleep architecture.
- Improves Quality of Life: Reduces daytime sleepiness, improves concentration, mood, and energy levels.
- Lowers Health Risks: Reduces long-term risks associated with untreated OSA, including hypertension, stroke, heart attack, and type 2 diabetes.
- Non-Invasive: Avoids the need for surgical intervention in many cases.
- Cost-Effective: Compared to the long-term healthcare costs of managing untreated OSA complications.
Limitations
- Adherence Dependency: Therapy is only effective if used consistently, every night, for the entire sleep period.
- Comfort Challenges: Initial discomfort with the mask and pressure is common and can lead to non-adherence.
- Not a Cure: CPAP manages the symptoms but does not cure the underlying anatomical cause of OSA.
- Power Dependent: Requires a consistent power source, though battery backups are available.
Safety Concerns & Warnings
- Electric Shock Hazard: Never use near water or with a damaged power cord.
- Fire Hazard: Do not use oxygen or flammable anesthetics in or near the device unless specified by the manufacturer.
- Contaminated Water: Use only distilled water in the humidifier to prevent mineral buildup and bacterial growth. Clean the chamber daily.
- Mask Leaks & Skin Irritation: Improper fit can cause eye irritation (from air leaks) or skin breakdown. Proper fitting and hygiene are crucial.
Contraindications
- Significant Respiratory Failure: Patients who cannot breathe adequately on their own may require a full ventilator.
- Recent Cranial Surgery or CSF Leak: The increased pressure could be dangerous.
- Untreated Pneumothorax: Air pressure could worsen a collapsed lung.
- Severe Bullous Lung Disease: Risk of air trapping and lung rupture.
- Extreme Anxiety or Claustrophobia: May prevent tolerance of the mask, though behavioral therapy and mask options can help.
5. Regulation
CPAP devices are regulated as moderate-to-high risk medical devices globally due to their life-supporting function.
- FDA Class: Class II (Special Controls). Requires a 510(k) premarket notification to demonstrate substantial equivalence to a legally marketed predicate device.
- EU MDR Class: Class IIa (for basic CPAP) or Class IIb (for devices with advanced features like auto-adjusting algorithms intended for vital physiological monitoring).
- CDSCO Category (India): Class C (Moderate to High Risk), equivalent to the US FDA’s Class II.
- PMDA Notes (Japan): Classified as “Specified Controlled Medical Devices” (Class II). They require certification from a Registered Certification Body (RCB) under the Pharmaceutical and Medical Device Act (PMD Act).
- ISO/IEC Standards:
- ISO 80601-2-70: Particular requirements for the basic safety and essential performance of sleep apnea breathing therapy equipment.
- ISO 17510: Sleep apnoea breathing therapy – Masks and application accessories.
- IEC 60601-1: General requirements for basic safety and essential performance of medical electrical equipment.
6. Maintenance
Proper maintenance is critical for efficacy, safety, and device longevity.
Cleaning & Sterilization
- Daily: Empty and rinse the humidifier chamber with warm water and mild soap. Air dry. Wash the mask cushion with soapy water.
- Weekly: Disassemble the mask, headgear, tubing, and chamber. Wash in warm water with mild dish soap. Rinse thoroughly. Air dry completely away from direct sunlight. Wipe the device exterior with a damp cloth.
- Sterilization: Components are not typically sterilized but sanitized. Some masks/tubing can be cleaned with a 1:1 white vinegar and water solution for 30 minutes, then rinsed. Always follow manufacturer guidelines.
Reprocessing
For single-patient use in home care, reprocessing is not applicable. In clinical settings where a device may be used for multiple patients, rigorous disinfection protocols per hospital policy and manufacturer IFU must be followed.
Calibration
The pressure calibration of CPAP blowers is factory-set and generally stable. It should be checked annually by an authorized service technician using a calibrated manometer to ensure it delivers the prescribed pressure accurately.
Storage
- Store in a clean, dry, well-ventilated area at room temperature.
- Keep away from dust, direct sunlight, and extreme temperatures.
- Store components disassembled and dry to prevent microbial growth.
- Protect from physical impact.
7. Procurement Guide
How to Select the Device
Consider the patient’s prescription (fixed vs. auto CPAP), clinical needs (need for humidification, data), and lifestyle (travel needs, noise sensitivity).
Quality Factors
- Clinical Efficacy: Proven performance in reducing AHI.
- Reliability & Durability: Mean time between failures, warranty length (typically 2-5 years).
- Patient Comfort Features: Ramp, pressure relief, advanced humidification.
- Data & Connectivity: Ease of data access for clinicians and patients via apps/portals.
- Noise Level: Should be very quiet (<30 dB).
Certifications
Look for CE Marking (EU), FDA 510(k) Clearance (US), and compliance with relevant ISO standards (80601-2-70). Country-specific marks like UKCA or BIS (India) may also be required.
Compatibility
Ensure compatibility with the hospital’s patient data management system (if used for monitoring) and availability of a range of mask interfaces from the same or third-party manufacturers to suit different patient facial structures.
Typical Pricing Range
- Device Only: $250 – $850 USD.
- Full Package (Device, Humidifier, Tubing, Mask): $500 – $1,200 USD.
- Note: Prices vary significantly by region, features, and whether purchased via insurance/DME or out-of-pocket.
8. Top 10 Manufacturers (Worldwide)
- ResMed (USA/Australia): Global leader in sleep and respiratory care. Notable lines: AirSense, AirCurve, AirMini.
- Philips Respironics (Netherlands/USA): A major historical player. Notable lines: DreamStation, System One. (Note: Check for recall status on older models).
- Fisher & Paykel Healthcare (New Zealand): Renowned for innovative humidification and mask interfaces. Notable lines: SleepStyle, Icon.
- DeVilbiss Healthcare (USA): Known for reliable, user-friendly devices. Notable line: IntelliPAP.
- 3B Medical (USA): Focus on ozone-free cleaning solutions and CPAP devices. Notable line: Luna.
- BMC Medical Co., Ltd (China): A growing global supplier of cost-effective CPAP and respiratory products.
- Apex Medical Corp. (Taiwan): Major OEM/ODM manufacturer and brand owner. Notable lines: iCH, XT.
- Medtronic plc (Ireland): Through its Covidien and earlier acquisitions, has a presence in the sleep and respiratory market.
- Drive DeVilbiss Healthcare (USA): Distinct from DeVilbiss, offering a range of DME including CPAP.
- Somnetics International, Inc. (USA): Manufacturer of the compact Transcend travel CPAP line.
9. Top 10 Exporting Countries (Latest Year – Based on Recent Trade Data Trends)
- China: The world’s manufacturing hub, exporting a vast volume of devices and components across all price segments.
- United States: Home to ResMed and Philips, exports high-value, technologically advanced units globally.
- Germany: A major European medical device exporter, with strong exports within the EU and beyond.
- Netherlands: Headquarters of Philips, a key export origin for their devices.
- Singapore: A major Asian logistics and trade hub for medical devices, including CPAPs from various manufacturers.
- Ireland: Hosts significant Medtronic and other med-tech manufacturing and distribution centers for export.
- Mexico: A growing manufacturing base for the North American market, exporting to the US and Latin America.
- New Zealand: Home to Fisher & Paykel, a significant exporter of premium devices and humidification systems.
- United Kingdom: Exports devices and has a strong domestic manufacturing base for certain brands.
- Switzerland: Known for high-precision medical device manufacturing and export.
10. Market Trends
Current Global Trends
- Shift to Telehealth & Remote Patient Monitoring (RPM): CPAP devices with cloud connectivity allow clinicians to monitor adherence and efficacy remotely, improving patient management.
- Rising Prevalence of OSA: Increasing awareness and diagnosis are driving market growth.
- Product Miniaturization: High demand for portable, travel-friendly devices.
New Technologies
- Advanced Algorithmic Therapy: Auto-adjusting algorithms are becoming more sensitive and comfortable.
- Integrated Pulse Oximetry: Some devices now connect to finger pulse oximeters for comprehensive sleep data.
- AI-Driven Insights: Artificial intelligence is being used to analyze therapy data and provide personalized tips for improving adherence.
Demand Drivers
- Aging global population (higher risk of OSA).
- Obesity epidemic (a primary risk factor for OSA).
- Increased consumer health awareness.
- Expansion of home-based healthcare.
Future Insights
The future points towards fully integrated sleep health ecosystems. CPAP devices will act as hubs, connecting with other wearables (smart rings, watches) to provide a holistic view of sleep health. Therapy will become even more personalized and automated, with predictive algorithms adjusting therapy preemptively. Competition will intensify, focusing on patient comfort, seamless user experience, and value-added digital services.
11. Training
Required Competency
- For Clinicians/Therapists: Ability to interpret sleep study data, titrate appropriate pressure settings, perform mask fittings, troubleshoot therapy problems, and educate patients.
- For Patients: Must be trained on device setup, daily use, cleaning, basic troubleshooting (leaks, condensation), and data interpretation via the app/display.
Common User Errors
- Incorrect Mask Fit: Leading to leaks, discomfort, and ineffective therapy.
- Neglecting Cleaning: Causing respiratory infections, skin irritation, and device malfunctions.
- Turning Off the Ramp Feature Prematurely: Making it harder to adapt to therapy.
- Using Tap Water in the Humidifier: Causing mineral buildup and bacterial growth.
- Inadequate Trial Period: Giving up on therapy before the crucial 2-4 week adaptation period.
Best-Practice Tips
- Mask First: Spend time finding the perfect mask. Comfort is 80% of the battle for adherence.
- Consistency is Key: Use the device every night, even for naps.
- Start Slow: Use the ramp feature and wear the mask while awake (e.g., watching TV) to acclimate.
- Regularly Review Data: Check your AHI and leak rate weekly to ensure therapy is effective.
- Replace Parts on Schedule: Follow manufacturer guidelines (mask cushion: monthly, tubing: 3 months, filter: monthly, etc.).
12. FAQs
1. Is CPAP therapy uncomfortable?
It can feel strange at first, but with the right mask fit, use of comfort features (like ramp), and a short adaptation period, most people find it very manageable and quickly appreciate the benefits of restful sleep.
2. Will I become dependent on the CPAP machine?
No. CPAP does not change your body’s ability to breathe. It’s like wearing glasses—you use them to correct a problem, but you don’t become “dependent” on them in a physiological sense. You may, however, become dependent on feeling well-rested!
3. How often do I need to clean my CPAP?
The mask cushion and water chamber should be rinsed daily. A full, thorough cleaning of all parts (tubing, mask, chamber) with soap and water should be done weekly.
4. Can I use tap water in the humidifier?
It’s strongly discouraged. Minerals in tap water can damage the chamber and be aerosolized into your lungs. Use distilled or demineralized water. In a pinch, boiled and cooled water is better than straight tap water.
5. What if I pull my mask off in my sleep?
This is common during the adjustment period. Try using the ramp feature, ensuring your mask isn’t too tight, and addressing any nasal congestion. Persistence usually solves this.
6. How long will it take to feel better?
Many people notice improved sleep and less daytime sleepiness within a few days. Full cognitive and cardiovascular benefits develop over weeks to months of consistent use.
7. Is it safe to use a CPAP when I have a cold?
Yes, it can actually help by keeping your airways open. You may need to use a full face mask if your nose is too congested, or temporarily increase humidity.
8. Can I travel with my CPAP?
Absolutely. All CPAPs are FAA-approved for carry-on use. Travel-specific models are even smaller. Always carry it as a medical device; it doesn’t count as a carry-on bag.
9. Why is my CPAP making a loud noise or whistling?
This is almost always caused by a leak. Check all connections (mask-to-tube, tube-to-device), ensure your mask is sealed properly, and listen along the tubing for holes.
10. How do I know if my pressure setting is correct?
Your doctor sets it based on your sleep study. Efficacy is confirmed by a low AHI (<5 events/hour) and low leak rate on your machine’s data. Report persistent fatigue or high AHI readings to your clinician.
11. Will my insurance cover a CPAP machine?
Most insurance plans, including Medicare, cover CPAP therapy with a qualifying sleep study diagnosis. Coverage details (rental vs. purchase) vary by plan.
12. What’s the difference between CPAP and APAP?
CPAP delivers one constant pressure. APAP automatically adjusts pressure within a set range based on your breathing. APAP is often preferred as it can provide more comfort and adapt to changes (like sleeping position, weight, alcohol use).
13. Conclusion
The CPAP device stands as a cornerstone of modern respiratory and sleep medicine. What began as a simple pneumatic solution for sleep apnea has evolved into a sophisticated, connected healthcare tool that saves and dramatically improves lives. Its success hinges not just on advanced technology but on a partnership between the patient, the clinician, and the device. Understanding its operation, applications, rigorous maintenance needs, and the broader market context is essential for anyone involved in its prescription, use, or procurement. As technology advances towards greater personalization and integration, CPAP therapy will become an even more seamless and powerful component of proactive health management, ensuring that a good night’s sleep remains within everyone’s reach.
14. References
- American Academy of Sleep Medicine (AASM). (2023). Clinical Practice Guidelines for the Treatment of Obstructive Sleep Apnea.
- U.S. Food and Drug Administration (FDA). (2023). Code of Federal Regulations Title 21, Sec. 868.5905 – Continuous Positive Airway Pressure (CPAP) Device.
- International Organization for Standardization (ISO). (2020). ISO 80601-2-70: Medical electrical equipment — Part 2-70: Particular requirements for the basic safety and essential performance of sleep apnea breathing therapy equipment.
- European Commission. (2023). Medical Device Regulation (MDR) – Annex VIII, Classification Rules.
- ResMed Clinical Guide. (2024). AirSense 11 Clinical Manual.
- Global Market Insights. (2024). Sleep Apnea Devices Market Size Report, 2024-2032.
- World Health Organization (WHO). (2022). Medical device technical series: technical specifications for respiratory care devices.
- Berry, R. B., et al. (2020). The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine.