1. Definition
What is a BiPAP/NIV Device?

A BiPAP (Bilevel Positive Airway Pressure) device, more broadly categorized as a Non-Invasive Ventilation (NIV) device, is a critical medical apparatus designed to support a patient’s breathing without the need for invasive intubation (inserting a tube into the windpipe). Think of it as a sophisticated, pressurized air pump. Its primary function is to assist or control a patient’s ventilation by delivering pressurized air through a sealed mask (covering the nose, mouth, or both). It provides two distinct levels of pressure: a higher pressure during inhalation (IPAP – Inspiratory Positive Airway Pressure) to help draw air into the lungs, and a lower pressure during exhalation (EPAP – Expiratory Positive Airway Pressure) to keep the airways open and make breathing out easier.
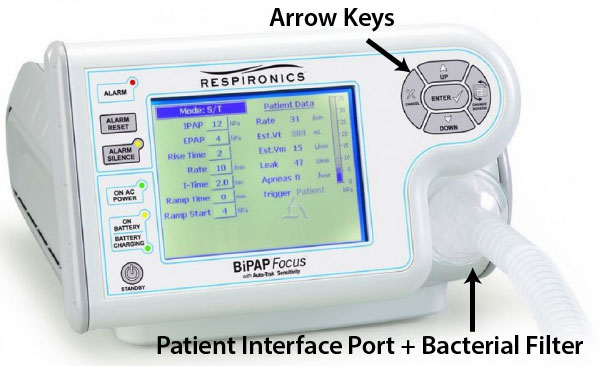
While “BiPAP” is a brand name (owned by Philips Respironics) that has become generic, it specifically refers to a mode of therapy. The broader term, Non-Invasive Ventilation (NIV), encompasses various modes including BiPAP, CPAP (Continuous Positive Airway Pressure), and ASV (Adaptive Servo-Ventilation). This guide focuses on the versatile, hospital-grade devices commonly used for acute and chronic respiratory failure.
How it Works
The device works on a simple but intelligent principle: pressure support. It is connected to a patient via a circuit and an interface (mask). A small, quiet internal turbine generates an adjustable flow of air.
- Detection: Sophisticated sensors detect the very beginning of the patient’s own attempt to inhale.
- Inhalation Support (IPAP): Upon detection, the device quickly ramps up to the prescribed higher pressure (IPAP). This pressure “boost” helps overcome airway resistance and poor lung elasticity, reducing the work of breathing. It’s like getting a push when you’re trying to pedal a bicycle up a hill.
- Exhalation Support (EPAP): When the device senses the end of inhalation, it drops to the lower pressure (EPAP). This pressure maintains a “splinting” effect on the alveoli (tiny air sacs) and airways, preventing them from collapsing. This makes exhalation less strenuous and improves gas exchange by allowing more carbon dioxide to be expelled.
Modern devices offer various modes (e.g., S, T, S/T, AVAPS) that tailor this pressure delivery—whether it’s triggered solely by the patient (Spontaneous), set at timed intervals (Timed), or a combination of both.
Key Components
- Main Unit/Blower: The core housing the turbine, motherboard, sensors, and control interface. It generates and regulates airflow.
- User Interface: A screen (LCD or touchscreen) and control knobs/buttons for setting parameters, viewing data, and alarms.
- Internal Battery: Provides backup power for portability and during power outages (typically 1-8 hours).
- Power Supply/Adapter: Converts AC power to DC for the unit and charges the battery.
- Air Filter: A reusable or disposable filter that prevents dust and particles from entering the blower.
- Breathing Circuit (Tubing): Flexible, lightweight tubing (usually 6-10 feet long) that delivers air from the device to the patient. Heated tubes are often used to prevent condensation (“rainout”).
- Patient Interface (Mask): The most crucial component for comfort and efficacy. Types include:
- Nasal Masks: Cover only the nose.
- Nasal Pillows/Cushions: Sit at the nostrils.
- Oronasal (Full Face) Masks: Cover both nose and mouth, essential for mouth-breathers or high-pressure therapy.
- Total Face Masks: Cover the entire face from forehead to chin.
- Humidifier (Integrated or Attached): A heated water chamber that warms and humidifies the delivered air, preventing nasal dryness, congestion, and mucosal injury. It is considered a standard of care for prolonged use.
2. Uses
Clinical Applications
BiPAP/NIV is a first-line intervention for several conditions characterized by acute or chronic respiratory failure. Its applications are broadly divided into two categories:
1. Acute Respiratory Failure:
- Hypercapnic Respiratory Failure (High CO2):
- Acute Exacerbation of COPD (AECOPD): The most well-established use. NIV reduces work of breathing, improves gas exchange, and significantly decreases the need for intubation and mortality.
- Acute Cardiogenic Pulmonary Edema: Works by increasing intrathoracic pressure, reducing preload and afterload on the heart, and improving oxygenation.
- Post-Extubation Respiratory Failure: Used to prevent re-intubation.
- Obesity Hypoventilation Syndrome (OHS) exacerbation.
- Neuromuscular Disease exacerbation (e.g., ALS, Myasthenia Gravis).
- Hypoxemic Respiratory Failure (Low O2):
- Community-Acquired Pneumonia (in selected patients).
- Immunocompromised patients (to avoid the risks of intubation).
- Palliative care for dyspnea relief.
2. Chronic Respiratory Management:
- Chronic Hypercapnic Respiratory Failure from restrictive thoracic diseases or neuromuscular disorders.
- Obstructive Sleep Apnea (OSA) in patients who cannot tolerate CPAP or have coexisting hypoventilation.
- Home Ventilation for patients with chronic conditions like muscular dystrophy or spinal cord injuries.
Who Uses It
- Physicians: Pulmonologists, Intensivists, Critical Care Specialists, Sleep Specialists, and Hospitalists prescribe settings and manage therapy.
- Respiratory Therapists (RTs): The primary experts in setting up the device, selecting interfaces, titrating pressures at the bedside, monitoring efficacy, and troubleshooting.
- Nurses (ICU, Ward, Home Care): Assist in patient monitoring, mask fitting, ensuring patient comfort, and managing the humidifier.
- Sleep Technologists: Use it during sleep studies for titration.
- Patients & Caregivers: In the home setting, trained patients and caregivers operate and maintain the device daily.
Departments/Settings
- Intensive Care Unit (ICU) & Critical Care: The most common acute setting.
- Emergency Department (ED): For rapid stabilization.
- Respiratory Wards/Step-down Units: For prolonged weaning and management.
- Sleep Laboratories: For diagnostic and titration studies.
- Home Healthcare: The fastest-growing segment for chronic management.
- Palliative Care Units: For symptom management.
3. Technical Specs
Typical Specifications
- Pressure Range: IPAP: 4-30 cm H₂O (some up to 40); EPAP: 4-25 cm H₂O.
- Breath Rate (for timed modes): 5-40 breaths per minute (BPM).
- Rise Time (Ti): Adjustable speed at which IPAP is reached.
- Sensitivity: Adjustable trigger (sensitivity to start inhalation) and cycle (sensitivity to switch to exhalation) settings.
- Alarms: High/Low pressure, apnea, low minute ventilation, high respiratory rate, circuit disconnect, power failure.
- Data Capabilities: Detailed event logging, compliance tracking (hours of use), leak estimation, AHI (Apnea-Hypopnea Index) reporting.
- Oxygen Port: Allows for blending of supplemental oxygen into the circuit.
- Dimensions & Weight: Varies; portable units: ~2-5 kg, 15x20x10 cm; hospital carts: larger with integrated batteries and monitors.
Variants & Sizes
- Hospital/ICU Ventilators with NIV Modes: High-end, multi-function ventilators.
- Stand-Alone Dedicated NIV Devices: The focus of this guide, used in wards and homecare.
- Portable/Travel Devices: Smaller, lighter, often with DC/battery focus for active lifestyles.
Materials & Features
- Construction: Durable medical-grade plastics (ABS, polycarbonate). Silicone and gel for mask cushions.
- Advanced Features:
- Auto-Tracking Algorithms (e.g., Auto-BiPAP): Automatically adjusts pressure within a set range based on detected events.
- AVAPS/iVAPS: Volume-assured modes that target a specific tidal volume, ideal for unstable conditions.
- Integrated Telemedicine: Cellular or Bluetooth connectivity for remote monitoring by clinicians.
- Heated Tubing with Climate Control: Automatically adjusts heat and humidity to prevent rainout.
- Mask Fit Check Feature: Guides the user to achieve an optimal seal.
Notable Models (Illustrative)
- **Philips Respironics V60 / F&P ** (Note: Recall history impacted some Philips models; always check for current compliant devices).
- ResMed Stellar 150 / AirCurve 10 series.
- Medtronic (Covidien) Puritan Bennett 560.
- Löwenstein Medical PrismaVISION / PrismaCR.
- Fisher & Paykel Healthcare SleepStyle (for OSA) / Vitera.
- Breas Vivo 65 / Z1 Auto.
- DeVilbiss IntelliPAP Bilevel.
- Apex Medical XT Series.
4. Benefits & Risks
Advantages
- Non-Invasive: Avoids complications of endotracheal intubation (e.g., ventilator-associated pneumonia, airway trauma, sedation needs).
- Improves Patient Outcomes: Reduces intubation rates, ICU length of stay, and mortality in specific conditions like COPD.
- Preserves Physiological Functions: Allows speaking, coughing, swallowing, and oral intake.
- Improves Comfort: Often better tolerated than invasive ventilation when patients are appropriately acclimatized.
- Cost-Effective: Reduces costs associated with ICU stay and intubation management.
- Portability: Enables early mobilization and transition to home care.
Limitations
- Mask-Related Issues: Discomfort, skin breakdown (bridge of nose), claustrophobia, and air leaks can compromise therapy.
- Patient Cooperation Required: Needs a conscious, cooperative patient with adequate airway protection (minimal aspiration risk).
- Limited Airway Access: Makes suctioning of secretions more difficult.
- Gastric Distension: Can cause bloating, though usually minor.
- Not for Complete Respiratory Failure: Cannot be used in apneic or severely obtunded patients without airway protection.
Safety Concerns & Warnings
- Airway Compromise: NIV is contraindicated in patients who cannot protect their airway (e.g., high aspiration risk, severely impaired consciousness).
- Undetected Disconnection: Alarms must be set and audible. A disconnected mask delivers no therapy.
- Barotrauma Risk: Although lower than invasive ventilation, excessive pressure can cause pneumothorax.
- Power/Battery Failure: Always have a backup plan and charged battery for critical patients.
- Infection Control: Circuits and filters must be changed per protocol to prevent bacterial colonization.
Contraindications
- Absolute: Cardiac/respiratory arrest, facial trauma/burns/deformity, recent upper GI/airway surgery, inability to fit mask, undrained pneumothorax.
- Relative: Hemodynamic instability, uncontrollable arrhythmias, agitated/uncooperative patient, excessive secretions, multi-organ failure.
5. Regulation
BiPAP/NIV devices are life-supporting and moderately high-risk, leading to strict regulatory oversight.
- FDA Class: Class II (Special Controls). Requires 510(k) premarket notification to demonstrate substantial equivalence to a predicate device.
- EU MDR Class: Class IIa (for treating sleep apnea) or Class IIb (for treating life-threatening respiratory insufficiency). Requires a conformity assessment by a Notified Body.
- CDSCO Category (India): Class C (Moderate to High Risk), aligned with the risk-based classification under the Medical Device Rules, 2017.
- PMDA (Japan): Classified as “Specified Controlled Medical Devices” (Class II). Requires marketing authorization (Shonin) from PMDA.
- ISO/IEC Standards:
- ISO 80601-2-12: The primary international standard specifying safety and performance requirements for critical care ventilators (includes NIV).
- ISO 80601-2-70: Particular requirements for ventilator-supplemental oxygen equipment.
- ISO 17510: For sleep apnea therapy devices.
- IEC 60601-1: General standard for basic safety and essential performance of medical electrical equipment.
6. Maintenance
Proper maintenance ensures device longevity, accuracy, and patient safety.
- Cleaning & Sterilization:
- Main Unit: Wipe exterior daily with a damp, soft cloth and mild detergent. Do not immerse in liquid.
- Patient Circuit & Mask: Clean daily with warm water and mild soap, rinse thoroughly, and air-dry out of sunlight. Replace per manufacturer schedule (typically every 3-6 months).
- Humidifier Chamber: Empty daily, clean with vinegar solution or recommended cleaner to prevent scaling, rinse, and air-dry.
- Filters: Check reusable foam filters weekly, wash gently, and air-dry. Replace disposable ultra-fine filters as per schedule (e.g., monthly).
- Reprocessing: Single-patient use circuits are standard. In hospital settings, some durable components may undergo high-level disinfection per institutional protocol.
- Calibration: Pressure and flow sensors require periodic calibration (e.g., annually) by authorized service technicians to ensure accuracy.
- Storage: Store in a clean, dry, well-ventilated area at room temperature. Protect from dust, extreme temperatures, and direct sunlight. Ensure the device is completely dry before storage.
7. Procurement Guide
How to Select the Device
Consider the primary care setting (ICU vs. home vs. sleep lab) and patient population (COPD vs. neuromuscular). Key questions:
- Do you need advanced volume-assured modes (iVAPS) or standard S/T?
- How important is portability and battery life?
- Is integrated telemedicine a requirement for home care?
- What is the availability of local service and technical support?
Quality Factors
- Performance & Accuracy: Consistent pressure delivery, sensitive trigger/cycle algorithms, low imposed work of breathing.
- Durability & Reliability: Robust construction, proven track record, low failure rate.
- Ease of Use: Intuitive interface for clinicians and patients, easy mask fitting procedure.
- Alarm System: Clear, configurable, and audible alarms.
- Data Reporting: Comprehensive, easy-to-interpret compliance and efficacy reports.
Certifications
Look for CE Marking (for EU), FDA 510(k) Clearance (for USA), and relevant regional certifications. ISO 13485 certification of the manufacturer’s quality management system is a strong indicator.
Compatibility
Ensure compatibility with your hospital’s preferred mask brands and circuits. Check if it integrates with existing patient monitoring systems or EMRs for data upload.
Typical Pricing Range
- Hospital-Grade Device (with basic humidifier): $1,500 – $4,000 USD.
- Homecare/Portable Device: $800 – $2,500 USD.
- Note: Masks ($50-$300), circuits ($30-$150), and accessories are recurring costs. Pricing varies significantly by region, volume, and features.
8. Top 10 Manufacturers (Worldwide)
- ResMed (USA/Australia): A global leader in sleep and respiratory care. Known for innovative algorithms, cloud connectivity (AirView), and user-friendly devices like the AirCurve series.
- Philips Respironics (Netherlands/USA): A historic leader with a vast portfolio. Notable for the V60 hospital ventilator and DreamStation series (subject to past recalls; verify current models).
- Fisher & Paykel Healthcare (New Zealand): Renowned for its world-class humidification technology and comfortable mask designs, used across its SleepStyle and Vitera platforms.
- Medtronic (Ireland/USA): Through its Covidien and Puritan Bennett divisions, it offers robust critical care and homecare ventilators with NIV capabilities.
- Löwenstein Medical (Germany): Highly regarded in Europe for premium, innovative devices like the Prisma line, featuring advanced therapy modes and detailed diagnostics.
- Getinge (Sweden): A major player in acute care with its Maquet ventilators (e.g., Servo-u) which include sophisticated NIV modes for the ICU.
- Dräger (Germany): Specializes in critical care and perioperative ventilators (e.g., Evita V series) with strong NIV functionality for hospital use.
- Breas Medical (Sweden): Focuses on home mechanical ventilation and sleep therapy, offering compact and versatile devices like the Vivo series.
- Apex Medical (Taiwan): A significant value-oriented global manufacturer offering a wide range of CPAP and BiPAP devices for the home care market (XT series).
- DeVilbiss Healthcare (USA): Provides a range of durable medical equipment, including the IntelliPAP BiPAP devices, often distributed through home care providers.
9. Top 10 Exporting Countries (Latest Year – Based on 2022-2023 Trend Data)
(Ranked by estimated export value of HS code 901920 – “Breathing appliances and gas masks”)
- Ireland: A major hub for medtech exports, hosting manufacturing for several top companies (e.g., Medtronic, ResMed).
- United States: Home to key players like ResMed (HQ), Philips Respironics, and a large domestic market driving production.
- Germany: A powerhouse of medical engineering, with exports from Löwenstein, Dräger, and Weinmann.
- China: A rapidly growing manufacturer and exporter of both high-spec and cost-effective devices.
- Netherlands: Hosts the headquarters of Philips, a central point for its global supply chain.
- Singapore: A key Asian distribution and manufacturing hub for multinational corporations.
- Switzerland: Home to Hamilton Medical and other niche ventilator manufacturers.
- Mexico: A crucial manufacturing location for the North American market, serving US companies.
- United Kingdom: Hosts R&D and manufacturing for companies like Smiths Medical.
- France: Home to manufacturers like Air Liquide Medical Systems.
10. Market Trends
- Current Global Trends: Explosive growth in home-based NIV post-pandemic, driven by cost-containment and patient preference. Rising prevalence of COPD and OSA is a steady demand driver. Increasing integration of telehealth and remote patient monitoring is becoming standard.
- New Technologies: Artificial Intelligence (AI) for predictive therapy adjustment and early exacerbation detection. Enhanced connectivity (4G/5G modules) for seamless data flow. Miniaturization leading to more discreet, travel-friendly devices.
- Demand Drivers: Aging global population, increasing obesity rates, growing awareness of sleep disorders, and healthcare systems prioritizing cost-effective outpatient care.
- Future Insights: The market will continue shifting from acute to home/community care. We will see more “all-in-one” devices that can provide multiple modes (CPAP, BiPAP, ASV, oxygen). Personalized medicine will drive algorithms that adapt in real-time to individual patient physiology. Competition from regional manufacturers in Asia will increase, offering more choices.
11. Training
Required Competency
Operators (RTs, Nurses) must understand:
- Respiratory physiology and the pathophysiology of conditions treated with NIV.
- Device mechanics, modes, and parameter definitions (IPAP, EPAP, Ti, Trigger).
- Mask selection and fitting techniques to minimize leak and pressure injury.
- Patient assessment: monitoring work of breathing, synchrony with the device, and interpreting blood gases/oximetry.
- Troubleshooting alarms and common problems.
Common User Errors
- Incorrect Mask Fit/Sizing: Leading to large leaks, eye irritation, and therapy failure.
- Improper Pressure Settings: Starting too high (causing intolerance) or too low (providing ineffective support).
- Neglecting Humidification: Causing nasal dryness and poor adherence.
- Ignoring Alarms: Simply silencing alarms without addressing the root cause (leak, displacement).
- Inadequate Patient Education: Not preparing the patient, leading to anxiety and refusal.
Best-Practice Tips
- “Start Low, Go Slow”: Initiate therapy with comfortable pressures and gradually titrate upwards as tolerated.
- Spend Time on Mask Fitting: The right mask is 50% of success. Offer different types.
- Use RAMP Feature: Allows the patient to fall asleep as pressure gradually increases.
- Check the Data: Regularly review device-reported compliance, leak, and AHI data to objectively assess therapy.
- Establish a Cleaning Routine: Patient adherence to cleaning prevents infections and equipment damage.
12. FAQs
1. What’s the difference between BiPAP and a ventilator?
BiPAP is a type of non-invasive ventilator. “Ventilator” is a broad term. Invasive mechanical ventilators require a tube in the trachea and are for the most critically ill. BiPAP is non-invasive, using a mask, and for patients who are breathing on their own but need support.
2. Can a patient talk or eat while on BiPAP?
Yes, they can talk, though their voice may sound muffled. Eating or drinking is generally not recommended while the mask is on due to a high risk of choking and aspiration. For long-term therapy, the mask is removed for meals.
3. How long can a patient stay on BiPAP?
In acute settings, it can be used continuously for days until the crisis resolves. For chronic use at home, it’s typically used during sleep for 4-8 hours per night, and sometimes during daytime naps.
4. What are the common side effects?
Mask discomfort, nasal dryness/congestion, skin redness, eye irritation (from leaks), abdominal bloating, and claustrophobia. Most are manageable with proper fitting, humidification, and acclimatization.
5. My patient is “fighting the ventilator.” What should I do?
First, check for causes: Leak, Obstruction (secretions), Anxiety/Pain, Failure (is the condition worsening?). Reassure the patient, check mask fit, adjust trigger sensitivity or rise time, and ensure pressures are appropriate. Sedation is a last resort in NIV.
6. How often should the filters be changed?
Disposable ultra-fine filters: every month or as indicated. Reusable foam filters: wash weekly and replace every 6 months, or as per manufacturer instructions. In dusty environments, check more frequently.
7. Can oxygen be connected to a BiPAP machine?
Yes, all hospital and most home BiPAP devices have a dedicated oxygen inlet port where O₂ tubing from a concentrator or tank can be connected. The device then delivers a blended air-oxygen mix.
8. Is BiPAP the same as CPAP?
No. CPAP delivers one constant pressure. BiPAP delivers two pressures—a higher one for inhale and a lower one for exhale. BiPAP provides more ventilatory support and is used for more complex conditions than CPAP (which is primarily for obstructive sleep apnea).
13. Conclusion
The BiPAP/NIV device is a cornerstone of modern respiratory care, effectively bridging the gap between simple oxygen therapy and invasive mechanical ventilation. Its power lies in its ability to provide significant physiological support while preserving patient comfort and avoiding the risks of intubation. Success hinges on a clear understanding of its indications and contraindications, meticulous attention to mask fitting and patient interface, and careful titration of settings by trained professionals. As technology advances, these devices are becoming smarter, more connected, and increasingly pivotal in managing chronic respiratory diseases at home. For clinicians, mastering NIV therapy is an essential skill that improves patient outcomes across a wide spectrum of clinical settings.
14. References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). (2024). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease.
- British Thoracic Society/Intensive Care Society. (2018). Guideline for the ventilatory management of acute hypercapnic respiratory failure in adults.
- International Organization for Standardization. (2020). ISO 80601-2-12:2020 Medical electrical equipment — Part 2-12: Particular requirements for basic safety and essential performance of critical care ventilators.
- U.S. Food and Drug Administration. (2024). Device Classification Panels.
- European Medicines Agency. (2023). Medical Device Regulation (MDR) 2017/745.
- Kacmarek, R. M., Stoller, J. K., & Heuer, A. J. (2020). Egan’s Fundamentals of Respiratory Care (12th ed.). Elsevier.
- ResMed, Philips Respironics, Fisher & Paykel Healthcare. (2023). Clinical Guides and Technical Manuals for respective devices.
- Grand View Research. (2024). Positive Airway Pressure (PAP) Devices Market Size, Share & Trends Analysis Report.