1. Definition
What is a Phacoemulsification System (Ophthalmic)?
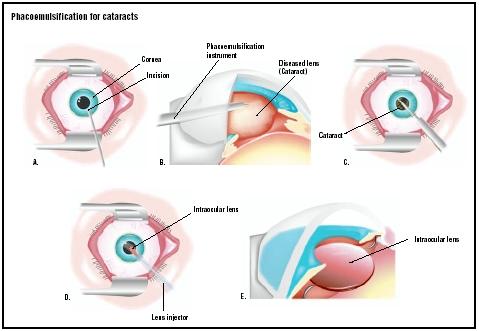
A phacoemulsification system, often called a “phaco machine,” is a sophisticated, microprocessor-controlled medical device used in ophthalmic surgery. Its primary function is to remove a clouded natural lens (cataract) from the eye through a very small incision, and then replace it with an artificial intraocular lens (IOL). The procedure, phacoemulsification, revolutionized cataract surgery by replacing large-incision manual techniques, enabling faster recovery and improved patient outcomes.
How it Works
In simple terms, the system uses ultrasonic energy to emulsify (break into tiny pieces) the hardened cataract. Here’s the basic workflow:
- Incision: The surgeon makes a tiny, self-sealing incision (typically 2-3 mm) in the cornea.
- Capsulorhexis: A circular opening is made in the front part of the lens capsule (the natural “bag” holding the lens).
- Emulsification: A handpiece with a vibrating titanium or steel needle (phaco tip) is inserted. The system generates controlled ultrasonic vibrations that break the lens into a fine emulsion.
- Aspiration: Simultaneously, an integrated fluidics system pumps a balanced salt solution into the eye to maintain its shape and pressure. The same system uses a vacuum (suction) to aspirate the emulsified lens particles out of the eye.
- Lens Implantation: Once the cataract is removed, a folded IOL is injected through the same small incision, unfolded, and placed into the empty lens capsule.
Key Components
- Main Console/Platform: The brain of the system, housing the computer, power supply, and control interfaces.
- Phacoemulsification Handpiece: Contains a piezoelectric crystal that vibrates the needle at ultrasonic frequencies (typically 28-45 kHz). Newer models may offer torsional, elliptical, or combination ultrasonic modes.
- I/A (Irrigation/Aspiration) Handpiece: A dual-port handpiece used after phaco to aspirate remaining lens cortex and polish the lens capsule.
- Fluid Management System:
- Irrigation: Uses a bottle of Balanced Salt Solution (BSS) suspended at a calculated height, or a pressurized cassette system, to maintain intraocular pressure (IOP).
- Aspiration Pump: Creates vacuum to remove fluid and lens material. Types include peristaltic, venturi, and diaphragm pumps.
- Foot Pedal: A multi-function controller that allows the surgeon to seamlessly switch between irrigation, aspiration, and ultrasonic power modes hands-free.
- Microscope and Video Integration: Modern systems integrate with the surgical microscope, overlaying critical parameters (power, vacuum, pressure) into the surgeon’s eyepiece or on a large screen.
- Advanced Software & Safety Systems: Software governs all parameters and includes safety features like surge suppression (to prevent rapid eye collapse) and thermal injury prevention at the incision site.
2. Uses
Clinical Applications
- Cataract Surgery: The primary and most common application for removing age-related, congenital, or traumatic cataracts.
- Refractive Lens Exchange (RLE): For correcting high refractive errors (nearsightedness, farsightedness) by removing the clear natural lens and replacing it with a prescriptive IOL.
- Management of Subluxated Lenses: For lenses that have become partially dislocated.
- Assisted Procedures: Can be used in certain cases of pediatric cataract or as a tool in complex anterior segment surgeries.
Who Uses It
- Ophthalmic Surgeons/Ophthalmologists: Specifically, cataract and anterior segment surgeons are the primary operators.
- Operating Room (OR) Nurses/Technicians: They are responsible for setting up the machine, priming the tubing, loading the correct fluids, assisting with handpiece preparation, and maintaining sterility.
- Biomedical Engineers/Technicians: Responsible for installation, periodic maintenance, calibration, and troubleshooting.
Departments/Settings
- Hospital Operating Theatres: Dedicated ophthalmic ORs.
- Ambulatory Surgery Centers (ASCs): The predominant setting for cataract surgery in many countries due to efficiency and cost-effectiveness.
- Specialized Ophthalmic Clinics/Hospitals: With in-house surgical facilities.
3. Technical Specifications
Typical Specifications
- Ultrasonic Frequencies: 28 kHz to 45 kHz, with adjustable power (0-100%).
- Aspiration Flow Rate (AFR): Adjustable, typically 0-60 mL/min.
- Vacuum Pressure: Adjustable, typically 0-600 mmHg (or higher for advanced systems).
- Irrigation Pressure: Manually set via bottle height (30-150 cm above eye) or controlled via console (cassette systems).
- Foot Pedal: 3-position (irrigation, irrigation+aspiration, irrigation+aspiration+ultrasound) or more with programmable modes.
- Dimensions & Weight: Varies by model; console typically 60-80 cm wide, 50-70 cm deep, and weighs 50-100 kg.
Variants & Sizes
Variants are primarily defined by their pump technology and ultrasound delivery:
- Peristaltic Pump: Offers precise control of flow rate; vacuum builds as tip is occluded. Favored for its controllability.
- Venturi Pump: Creates instantaneous vacuum; vacuum level is the primary control. Known for high efficiency.
- Torsional/Elliptical Ultrasound: Side-to-side or elliptical tip motion, which can be more efficient and generate less heat than traditional longitudinal (jackhammer) ultrasound.
Materials & Features
- Materials: Consoles use medical-grade plastics and metals. Handpieces and tips are titanium or stainless steel. Single-use consumables (kits, tubing) are medical-grade plastics.
- Special Features:
- Surge Suppression: Software algorithms to prevent post-occlusion surge.
- Thermal Monitoring: To prevent corneal burn at the incision.
- Fluidics Management Systems: Advanced systems (e.g., Alcon’s Active Sentry, J&J’s Stable chamber) actively monitor and control pressure.
- Femtosecond Laser Integration: Some systems interface with femtosecond lasers for laser-assisted cataract surgery (LACS/FLACS).
- Modularity & Upgradeability: Ability to add new software or hardware modules.
Notable Models (Examples)
- Alcon (USA): Centurion Vision System, LenSx Laser (FLACS), Infiniti Vision System (legacy, widely used).
- Johnson & Johnson Vision (USA): WhiteStar Signature PRO, INTREPID (hybrid venturi/peristaltic).
- Bausch + Lomb (USA): Stellaris Elite, Stellaris PC (modular).
- Carl Zeiss Meditec (Germany): CALLISTO eye (for alignment), often paired with a phaco unit.
- NIDEK (Japan): Cataract & Refractive Suite.
4. Benefits & Risks
Advantages
- Minimally Invasive: Small incisions (often sutureless) lead to rapid healing and less induced astigmatism.
- Improved Patient Outcomes: Faster visual recovery, less postoperative discomfort, and excellent refractive results.
- Enhanced Surgeon Control: Programmable settings and advanced fluidics allow for customization to surgical technique and cataract density.
- Efficiency: Surgery times are often under 20 minutes, optimizing OR throughput.
- Versatility: Can handle a wide range of cataract densities.
Limitations
- High Initial Cost: The capital investment for a new system is significant.
- Learning Curve: Surgeons require specific training to master the technique, especially managing fluidics and energy.
- Consumable Costs: Ongoing expense for single-use procedural packs (tubing, cassettes, sleeves).
- Not Suitable for All Cataracts: Extremely dense, rock-hard (brunescent) cataracts may require alternative techniques.
Safety Concerns & Warnings
- Thermal Injury: Prolonged ultrasound with restricted irrigation can heat the phaco tip, causing a corneal burn (incisional burn).
- Post-Occlusion Surge: Sudden release of occlusion can cause rapid collapse of the anterior chamber, risking damage to the posterior capsule or iris.
- Incorrect Settings: Improperly set vacuum, power, or bottle height can lead to complications like capsule rupture or corneal edema.
- Infection Risk: Breach in sterile technique during setup or use.
Contraindications
The procedure, not the device itself, may be contraindicated in patients with:
- Advanced corneal disease where visualization is poor.
- Extremely unstable zonules (ligaments holding the lens) where capsule support is insufficient.
- Active ocular infection (e.g., endophthalmitis). Relative contraindications include very dense cataracts or certain patient co-operability issues.
5. Regulation
- FDA Class: Class II (Special Controls). These devices are subject to premarket notification (510(k)) and performance standards.
- EU MDR Class: Class IIb. Devices for diagnosis, monitoring, or correction of a defect of the heart or central circulatory system or central nervous system. Phaco falls under “invasive devices for use in ophthalmology.”
- CDSCO Category (India): Class C (Moderate to High Risk), equivalent to a risk class higher than US FDA Class II.
- PMDA (Japan): Classified as “Controlled Medical Devices” (Class III or IV), requiring a high level of review and approval. MHLW Ordinance 169 governs their certification.
- ISO/IEC Standards:
- ISO 80601-2-58: Particular requirements for the basic safety and essential performance of lens removal devices and vitrectomy devices for ophthalmic surgery.
- ISO 13485: Quality management systems for medical devices.
- IEC 60601-1: General requirements for basic safety and essential performance of medical electrical equipment.
6. Maintenance
Cleaning & Sterilization
- Console/Exterior: Clean daily with a soft cloth dampened with mild detergent or hospital-grade disinfectant (not abrasive or alcohol-based).
- Handpieces (Reusable): Must be cleaned and sterilized after each use per manufacturer’s IFU. Typically involves:
- Immediate wiping to remove gross debris.
- Flushing channels with water or enzymatic cleaner.
- Ultrasonic cleaning.
- Thorough rinsing and drying.
- Steam autoclaving (check IFU for specific cycle type).
Reprocessing
- Single-Use Items: Phaco packs, tubing sets, cassettes, and sleeves are strictly for single use. Reprocessing them is unsafe and violates regulations.
- Handpiece Reprocessing: Follow the validated IFU meticulously. Inadequate cleaning can lead to bio-burden buildup, clogging, and performance failure.
Calibration
- Must be performed regularly (e.g., annually or per manufacturer schedule) by qualified biomedical engineers or authorized service personnel.
- Includes verification and adjustment of ultrasound power output, vacuum/pressure sensors, aspiration flow rates, and foot pedal responsiveness.
Storage
- Store in a clean, dry, temperature-controlled environment (specified in IFU, typically 10-40°C).
- Protect from dust, moisture, and direct sunlight.
- Handpieces should be stored sterile in their containers.
- Keep all cables and tubing neatly coiled to prevent damage.
7. Procurement Guide
How to Select the Device
- Assess Surgical Volume & Case Mix: High-volume ASCs need speed and reliability. Complex case practices may prioritize advanced fluidics and safety features.
- Evaluate Technology: Consider pump type (peristaltic vs. venturi), ultrasound modalities (torsional, longitudinal), and integration capabilities (with microscope, laser).
- Test Ergonomics: Surgeon comfort with the foot pedal, screen layout, and handpiece balance is critical.
- Analyze Total Cost of Ownership (TCO): Include initial price, service contract costs, and per-procedure consumable costs, which are a major recurring expense.
Quality Factors
- Reliability & Uptime: Reputation for low failure rates.
- Service & Support: Quality, speed, and local availability of technical support.
- Ease of Use: Intuitive interface for both surgeons and staff.
- Safety Features: Robustness of surge suppression, thermal protection, and system alarms.
Certifications
Ensure the device has the necessary regulatory clearances for your region: FDA 510(k) Clearance, CE Marking (under MDR), PMDA certification, CDSCO license as applicable.
Compatibility
- Check compatibility with your existing operating microscope (for data overlay).
- Verify compatibility with preferred IOL delivery systems and single-use consumables.
- Consider hospital/ASC IT integration for data recording and EMR connectivity.
Typical Pricing Range
- High-End System (e.g., Alcon Centurion, J&J INTREPID): $80,000 – $150,000+ USD.
- Mid-Range System (e.g., B+L Stellaris PC): $50,000 – $90,000 USD.
- Consumable Cost per Procedure: $150 – $400 USD (for single-use pack, sleeves, tips).
- Note: Pricing is highly negotiable and often bundled with multi-year service contracts and consumable purchase agreements.
8. Top 10 Manufacturers (Worldwide)
- Alcon (USA/Switzerland): The undisputed market leader. Renowned for its Centurion and legacy Infiniti systems, and integration with the LenSx femtosecond laser.
- Johnson & Johnson Vision (USA): A major competitor with the WhiteStar Signature PRO and INTREPID systems, known for innovative fluidics.
- Bausch + Lomb (USA): Offers the versatile Stellaris platform, known for its modularity and value proposition.
- Carl Zeiss Meditec (Germany): A leader in visualization (microscopes) and FLACS, with integrated solutions that often feature phaco units.
- NIDEK (Japan): A strong global player with comprehensive ophthalmic portfolios, including the Cataract & Refractive Suite.
- Topcon Corporation (Japan): Known for diagnostic devices, also manufactures and distributes phaco systems in various markets.
- Appasamy Associates (India): A leading Indian ophthalmic equipment manufacturer offering indigenously developed phaco systems at competitive prices.
- SurgiMac (India): Another significant Indian manufacturer providing cost-effective phacoemulsification systems.
- Möller-Wedel GmbH (Germany) (acquired by Haag-Streit): Primarily a microscope manufacturer, but its surgical units often include integrated phaco.
- Optikon (Italy): A well-established European manufacturer of ophthalmic surgical equipment, including phaco systems.
9. Top 10 Exporting Countries (Latest Data Trends)
Based on analysis of medical device export data for ophthalmic instruments (HS Code 901850):
- United States: Dominant exporter, home to Alcon, J&J, B+L. Leads in high-value, advanced systems.
- Germany: Major hub for precision engineering (Zeiss, Möller-Wedel). High-value exports.
- Japan: Strong exporter of reliable, high-tech systems (NIDEK, Topcon).
- Switzerland: Includes exports from Alcon’s operational HQ and other precision medtech.
- Ireland: A significant medtech export hub due to the presence of many US manufacturers’ European bases.
- France: Home to several specialized medtech companies serving the ophthalmic sector.
- China: Growing rapidly as a manufacturer and exporter of mid-range and value segment systems.
- India: Emerging as a key exporter of cost-effective systems to Asia, Africa, and Latin America (Appasamy, SurgiMac).
- Italy: Exports from companies like Optikon and other specialized manufacturers.
- United Kingdom: Exports of niche ophthalmic surgical technologies.
10. Market Trends
- Current Global Trends:
- Rising Cataract Prevalence: Driven by aging populations globally, fueling demand.
- Growth of Ambulatory Surgery Centers (ASCs): The shift from hospital to ASC-based surgery drives procurement of efficient, user-friendly systems.
- Premiumization: Surgeons and facilities seek advanced systems that enable premium IOL (multifocal, toric) implantation with better outcomes.
- New Technologies:
- Femtosecond Laser-Assisted Cataract Surgery (FLACS): Integration of laser for key steps (incisions, capsulorhexis, lens fragmentation).
- Advanced Fluidics & Safety: AI-driven pressure control and real-time data analytics.
- Energy Modulation: Continued evolution of non-longitudinal ultrasound (torsional, elliptical) for efficiency and safety.
- Demand Drivers:
- Aging demographics.
- Increasing patient expectations for refractive outcomes.
- Technological advancements.
- Improving healthcare access in emerging economies.
- Future Insights:
- Artificial Intelligence (AI): AI for pre-operative planning, real-time surgical guidance (e.g., capsulorhexis size, incision placement), and complication prediction.
- Enhanced Connectivity: Seamless data flow to EMRs and surgical video management systems.
- Robotics: Early-stage development of robotic-assisted platforms for extreme precision.
11. Training
Required Competency
- Surgeons: Formal ophthalmology residency, followed by cataract surgery-specific fellowship or supervised training. Includes didactic learning, wet-lab practice on animal/artificial eyes, and graded surgical experience.
- OR Staff: Manufacturer-provided in-service training on setup, priming, trouble-shooting alarms, and maintaining sterility. Certified ophthalmic assistant (COA) or similar training is beneficial.
Common User Errors
- Incorrect Setup/Tubing: Reversing irrigation and aspiration lines, improper cassette loading, or failing to prime lines (creating air bubbles).
- Ignoring Alarms: Overriding or not understanding system alarms for occlusion, low irrigation, or high pressure.
- Inappropriate Settings: Using excessively high power or vacuum for the cataract density, increasing complication risk.
- Poor Foot Pedal Control: “Riding the pedal” in phaco mode, leading to excessive energy use.
Best-Practice Tips
- For Staff: Always perform a pre-use check. Follow the priming protocol meticulously. Keep a log of system performance and maintenance.
- For Surgeons: Master foot pedal control. Start with lower parameters and increase as needed. Use pulsed or burst phaco modes to reduce total energy delivery. Respect the fluidics—maintain chamber stability above all.
12. FAQs
1. What’s the difference between peristaltic and venturi pumps?
Peristaltic pumps control flow rate to build vacuum, offering precision. Venturi pumps control vacuum directly, offering rapid response. Choice is based on surgeon preference and technique.
2. How long does a phaco handpiece last?
With proper care and sterilization, a reusable metal handpiece can last for thousands of procedures (typically 500-1000+), but the internal crystals can degrade. Follow the manufacturer’s lifespan guidelines.
3. Can a phaco machine be used for a “dropped nucleus”?
The I/A handpiece can be used to manage small fragments, but for a large dropped nucleus into the vitreous, a pars plana vitrectomy setup is required.
4. Why is surge suppression so important?
Post-occlusion surge is a dangerous vacuum release that can collapse the anterior chamber, potentially causing posterior capsule rupture or iris damage. Modern systems have sophisticated software to prevent it.
5. How often does the system need calibration?
Typically annually, but always follow the manufacturer’s recommended preventive maintenance schedule, which may be based on usage hours.
6. Can we use generic/third-party consumables?
Only if they are explicitly cleared for use with your specific model. Using non-compatible consumables can void warranties, cause malfunctions, and pose patient safety risks.
7. What is torsional phaco?
Instead of the traditional “jackhammer” in-and-out motion, the tip oscillates in a side-to-side arc. This can be more efficient at cutting and generates less heat.
8. Is phacoemulsification painful for the patient?
No. The procedure is performed under local anesthesia (drops or a small injection) with sedation. The patient is awake but feels no pain.
13. Conclusion
The phacoemulsification system stands as one of the most impactful technological advancements in modern surgery. By enabling cataract removal through a tiny, sutureless incision, it has transformed a once-major procedure into a highly efficient, outpatient surgery with exceptional patient outcomes. From its core components—ultrasonic handpiece, fluidics, and pump—to the cutting-edge trends of AI and laser integration, understanding this system is crucial for any ophthalmic practice. Successful adoption hinges not only on selecting the right technology but also on investing in comprehensive training, diligent maintenance, and a commitment to patient safety at every step.
14. References
- American Academy of Ophthalmology. (2023). Basic and Clinical Science Course (BCSC), Section 11: Lens and Cataract.
- U.S. Food and Drug Administration (FDA). (n.d.). Device Classification Database.
- European Commission. (2017/745). Medical Device Regulation (MDR).
- International Organization for Standardization. (2014). ISO 80601-2-58: Medical electrical equipment – Part 2-58: Particular requirements for the basic safety and essential performance of lens removal devices and vitrectomy devices for ophthalmic surgery.
- Devgan, U. (2020). Phacoemulsification: Strategies for Complicated Cataracts. Slack Incorporated.
- Market research reports from credible firms (e.g., Grand View Research, MarketScope) on the global ophthalmic surgical devices market.
- Manufacturer Instructions for Use (IFU) for Alcon Centurion, Johnson & Johnson INTREPID, and Bausch + Lomb Stellaris systems.