1. Definition
What is an Anesthesia Machine?
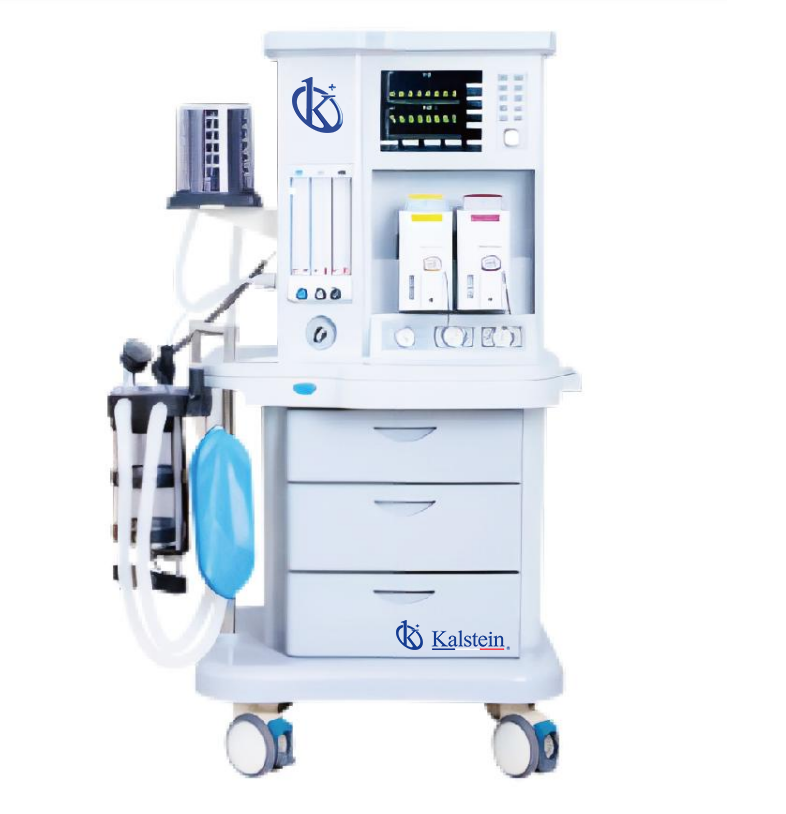
An anesthesia machine, often referred to as an anesthesia workstation, is a sophisticated medical device designed to deliver a precise and controlled mixture of medical gases (primarily oxygen and anesthetic agents) to a patient while simultaneously removing carbon dioxide during surgical procedures. Its primary function is to maintain general anesthesia—a reversible state of unconsciousness, analgesia, muscle relaxation, and amnesia—allowing patients to undergo surgery safely and comfortably. Think of it as a life-support system and a drug delivery system combined; it mechanically ventilates the patient while precisely dosing potent anesthetic vapors.
How it Works
The fundamental principle of an anesthesia machine is continuous gas flow and patient ventilation. Here’s a simplified breakdown:
- Gas Supply: Medical gases (O₂, medical air, nitrous oxide) are supplied from central hospital pipelines or onboard cylinders (E-cylinders) at high pressure.
- Pressure Regulation & Blending: The machine reduces this high pressure to a safe, usable level. The oxygen and other gases are then blended in precise ratios. A vital safety feature is the oxygen failure protection device, which ensures other gases cannot be delivered if oxygen pressure fails.
- Vaporization: The blended gas flow is directed through a vaporizer. Here, a specific concentration of a liquid anesthetic agent (like sevoflurane or desflurane) is converted into vapor and added to the gas mixture.
- Delivery & Ventilation: This final anesthetic gas mixture is delivered to the patient via a breathing circuit (a series of tubes). The machine’s ventilator (or a manual reservoir bag) breathes for the patient, pushing the gas into the lungs.
- Scavenging & Monitoring: The patient exhales, and the breathing circuit directs the exhaled gas, now containing CO₂, through a carbon dioxide absorbent (like soda lime) to remove the CO₂. The “cleaned” gas can then be re-breathed (in a circle system), conserving anesthetic and heat/humidity. Excess gas is safely scavenged to the outside. Throughout this process, integrated monitors continuously track patient vital signs and machine parameters (like oxygen concentration, airway pressure, and anesthetic agent levels).
Key Components
- Gas Supply System: Includes hoses, connectors, pressure gauges, and regulators for pipeline and cylinder gases.
- Flowmeters (Rotameters): Allow precise manual adjustment of the flow rate (in L/min) for each gas.
- Vaporizers: Device-specific units that accurately vaporize and meter a controlled concentration of anesthetic liquid into the carrier gas flow.
- Breathing Circuit: The pathway for gases to and from the patient. Most modern machines use a circle system with inspiratory and expiratory limbs, unidirectional valves, a Y-piece, and a reservoir bag.
- Ventilator: A mechanical bellows or piston that automatically delivers breaths to the patient, with adjustable settings for volume, pressure, rate, and inspiratory/expiratory ratios.
- Scavenging System: Collects and removes waste anesthetic gases from the circuit to protect the operating room staff.
- Monitoring & Alarm Systems:
- Oxygen Analyzer: The most critical monitor, placed in the breathing circuit.
- Airway Pressure Monitor: Measures pressure in the patient’s airway.
- Capnography: Measures exhaled CO₂ concentration—a key indicator of ventilation and metabolism.
- Spirometry: Measures inspired and expired gas volumes.
- Anesthetic Agent Analyzer: Identifies and measures the concentration of anesthetic gases.
- Integrated alarms for hypoxic mixtures, low pressure, apnea, and high airway pressure.
2. Uses
Clinical Applications
- General Anesthesia: The primary application for conducting surgery across all specialties—from orthopedics to neurosurgery.
- Sedation: For procedures outside the OR, like in endoscopy suites or interventional radiology, where lower levels of sedation are required.
- Mechanical Ventilation: Can serve as a backup or transport ventilator in critical care settings (e.g., ICU, during hospital transfers).
- Critical Care: Some advanced workstations are used in ICUs for long-term ventilation of critically ill patients.
- Patient Resuscitation: Provides a reliable source of oxygen and ventilation support during cardiopulmonary resuscitation.
Who Uses It
- Anesthesiologists & Anesthetists: The primary operators who manage all aspects of the machine and anesthesia delivery.
- Anesthesia Assistants & Nurses: Assist in machine setup, checking, and monitoring during procedures.
- Critical Care Physicians & Respiratory Therapists: May utilize the machine’s ventilator function in ICU settings.
- Biomedical Engineers/Technicians: Responsible for installation, preventive maintenance, calibration, and repairs.
Departments/Settings
- Operating Rooms (ORs): The most common setting.
- Day Surgery/Ambulatory Surgery Centers.
- Delivery Rooms/Labor & Delivery: For providing anesthesia during cesarean sections.
- Intensive Care Units (ICUs) & Critical Care Units.
- Cardiac Catheterization Labs & Interventional Radiology Suites.
- Emergency Departments (in some configurations).
- MRI Suites: Using MRI-compatible anesthesia machines.
3. Technical Specs
Typical Specifications
- Dimensions: Varies by model; typically ~100-130 cm H x 50-70 cm W x 60-80 cm D.
- Weight: 100-200 kg.
- Power Supply: 100-240V AC, 50/60 Hz. Equipped with internal battery backup (30 mins to several hours).
- Gas Inlets: For O₂, N₂O, medical air (typically at 45-60 psi/3-4 bar pipeline pressure).
- Gas Cylinder Yokes: For backup E-cylinders of O₂ and N₂O.
- Ventilator: Tidal Volume range: 20-1500 mL. Respiratory Rate: 2-80 bpm. Multiple ventilation modes (Volume Control, Pressure Control, SIMV, etc.).
- Integrated Monitors: As described in Key Components.
Variants & Sizes
- Standard Operating Room Workstations: Full-featured, integrated systems.
- Compact/Portable Machines: For MRI suites, remote locations, or field hospitals.
- ICU Ventilator-Anesthesia Combos: Designed for prolonged use in critical care.
- Draw-Over Anesthesia Devices: Simple, non-electric devices for extreme resource-limited settings.
Materials & Features
- Construction: High-impact plastics, stainless steel, and aluminum for durability and cleanability.
- Touchscreen Displays: Large, color, intuitive user interfaces for monitoring and control.
- Integrated Gas Monitoring: Often using Raman spectroscopy or infrared absorption.
- Advanced Ventilation Modes: Pressure support, lung-protective strategies.
- Electronic Gas Mixing: Replaces traditional mechanical flowmeters for greater precision.
- Data Integration & Connectivity: Ethernet, USB ports for electronic anesthesia record keeping (EARK) and hospital information systems (HIS).
- Low-Flow & Minimal-Flow Anesthesia Capability: To reduce agent consumption and environmental impact.
Notable Models
- GE Datex-Ohmeda: Aisys®, Aespire®, Avance®, Carescape®.
- Dräger: Perseus®, Apollo®, Zeus®, Fabius® series.
- Mindray: A-Series (A5, A7), Wato® series.
- Spacelabs: ARKON™.
- Maquet: Flow-i.
4. Benefits & Risks
Advantages
- Patient Safety: Enables precise control over oxygenation, ventilation, and anesthesia depth.
- Integrated Monitoring: Provides a comprehensive, real-time view of patient and machine status.
- Efficiency: Automated ventilation frees the anesthetist for other tasks. Advanced features streamline workflow.
- Versatility: Can be used for a wide range of patients, from neonates to adults, and for various procedures.
- Cost-Effectiveness: Modern low-flow capabilities significantly reduce the consumption of expensive anesthetic gases.
Limitations
- Complexity: Requires extensive training to operate safely.
- Dependence on Electricity & Gas Supply: Malfunctions in utilities can cripple the device without proper backups.
- Size & Portability: Standard machines are not easily mobile.
- High Initial Cost: Acquisition and installation are significant investments.
Safety Concerns & Warnings
- Hypoxic Mixture Risk: Malfunction can lead to delivery of a gas mixture with insufficient oxygen.
- Ventilator Failure: Can lead to apnea or barotrauma.
- Anesthetic Overdose: Due to vaporizer malfunction or user error.
- Circuit Leaks or Disconnections: A major cause of critical incidents.
- Failure to Scavenge: Leads to occupational exposure to waste gases.
- Pre-use Checkout is Mandatory: A comprehensive safety check (following a checklist like the FDA/ASA protocol) must be performed before each use to prevent most hazards.
Contraindications
There are no direct patient contraindications to the use of an anesthesia machine, as it is the tool for delivering a required medical state. However, specific components or modes may be contraindicated:
- Using a desflurane vaporizer without an electrical power supply (it requires heat to vaporize).
- Using nitrous oxide in patients with pneumothorax, bowel obstruction, or certain types of ear or eye surgery.
- Certain ventilation modes may be unsuitable for specific lung pathologies (e.g., severe ARDS).
5. Regulation
Anesthesia machines are high-risk, life-supporting devices and are heavily regulated worldwide.
- FDA Class: Class II (special controls). They require Premarket Notification [510(k)] demonstrating substantial equivalence to a predicate device.
- EU MDR Class: Class IIb (devices for supplying energy in the form of radiation/vital substances, and devices for controlling/monitoring such devices).
- CDSCO Category (India): Class C (moderate to high risk), requiring a manufacturing license and prior approval for import/manufacture.
- PMDA (Japan): Classified as Class II Controlled Medical Devices. They require certification from a Registered Certification Body (RCB).
- ISO/IEC Standards:
- ISO 80601-2-13: The core standard for “Medical electrical equipment – Part 2-13: Particular requirements for basic safety and essential performance of an anaesthetic workstation.”
- ISO 8835-3: Specifications for anaesthetic gas monitors.
- ISO 5358: Specification for agent-specific vaporizers.
- IEC 60601-1: General standard for the safety of medical electrical equipment.
6. Maintenance
Cleaning & Sterilization
- External Surfaces: Clean daily and after each case with a hospital-grade, non-abrasive disinfectant. Avoid bleach on screens.
- Breathing Circuit: Single-use, disposable circuits are standard. They are used for one patient and discarded.
- Reusable Components (e.g., certain ventilator bellows): Must be removed and sterilized according to manufacturer’s instructions, typically using low-temperature hydrogen peroxide gas plasma or ethylene oxide (EtO) sterilization. Never autoclave parts unless explicitly specified.
Reprocessing
Refers to the steps between patients:
- Discard all single-use items (circuit, CO₂ absorbent, filters).
- Wipe down the entire machine, paying attention to touch points.
- Attach a new, clean breathing circuit and fresh CO₂ absorbent.
- Perform the pre-use checkout procedure.
Calibration
- Gas Monitors (O₂, CO₂, agents): Require regular calibration (often daily or per manufacturer schedule) using known standard gas mixtures (calibration gas).
- Vaporizers: Must be serviced and calibrated annually by certified technicians.
- Flowmeters & Ventilators: Calibrated during scheduled preventive maintenance (every 6-12 months).
Storage
- Store in a clean, dry, temperature-controlled environment.
- Ensure the machine is secured to prevent tipping.
- When not in use for prolonged periods, disconnect from gas supplies, turn off, and charge the battery fully periodically.
7. Procurement Guide
How to Select the Device
- Assess Clinical Needs: Consider patient volume, types of surgeries (adult, pediatric, cardiac), and need for portability (MRI).
- Evaluate Workflow Integration: How well does the user interface fit your team’s workflow? Does it integrate with your EARK/HIS?
- Consider Future-Proofing: Look for modular designs that allow for hardware/software upgrades.
Quality Factors
- Reliability & Uptime: Check mean time between failures (MTBF).
- Safety Record & Alarms: Comprehensiveness and intuitiveness of the alarm system.
- Ease of Use & Training: Intuitive design reduces errors.
- Service & Support: Quality and responsiveness of the local service network.
Certifications
Look for CE Marking (for EU), FDA Clearance (for USA), and other regional certifications specific to your country (e.g., CDSCO in India, NMPA in China).
Compatibility
- Gas Pipeline Connections: Must match your hospital’s DISS (Diameter Index Safety System) or NIST outlets.
- Data Ports: Compatibility with your hospital’s data network for electronic records.
- Accessories: Availability and cost of compatible consumables (circuits, filters, absorbent).
Typical Pricing Range
Wide range based on features:
- Basic Model: $15,000 – $40,000
- Mid-Range Workstation: $40,000 – $80,000
- High-End Integrated Workstation: $80,000 – $150,000+
8. Top 10 Manufacturers (Worldwide)
- Drägerwerk AG & Co. KGaA (Germany): A global leader. Known for innovation, quality, and comprehensive portfolios (Perseus, Apollo).
- GE HealthCare (USA): Formed from GE and Datex-Ohmeda. A dominant player with the Carescape and Aisys platforms.
- Mindray Bio-Medical Electronics Co., Ltd. (China): A rapidly growing global contender offering high-value, feature-rich machines (A-Series).
- Getinge AB (Sweden): Through its Maquet division, produces the popular Flow-i and Flow-c series.
- Spacelabs Healthcare (USA): A subsidiary of OSI Systems, known for its ARKON anesthesia delivery system.
- Medtronic plc (Ireland): Offers anesthesia solutions, though more focused on patient monitoring and ventilators.
- Heinen + Löwenstein (Germany): Specializes in anesthesia workstations and critical care ventilators (Leon series).
- Beijing Aeonmed Co., Ltd. (China): A significant manufacturer supplying globally, known for cost-effective and reliable devices.
- Mermaid Care (Norway/Denmark): Focuses on simplified, intuitive anesthesia solutions like the Mermaid.
- Penlon Ltd. (UK): A historic manufacturer with a strong presence, particularly in the UK and Commonwealth markets (Primus, Sigma).
9. Top 10 Exporting Countries (Latest Year)
(Based on HS Code 9018 – Medical instruments, likely including anesthesia apparatus, and recent trade analysis)
- Germany: The world’s leading exporter of high-end medical devices, driven by Dräger and a strong engineering base.
- United States: Major exporter of advanced technology workstations from GE HealthCare and others.
- China: Fast-growing export volume, led by Mindray and Aeonmed, offering competitive mid-range devices.
- Ireland: A key export hub for Medtronic and other multinationals with manufacturing bases there.
- Sweden: Home to Getinge/Maquet, a significant exporter in Europe and beyond.
- United Kingdom: Exports specialized equipment from companies like Penlon.
- Japan: Exports high-quality devices from manufacturers like Fukuda Denshi and Nihon Kohden.
- Italy: Has a strong domestic medical device sector with export capabilities.
- France: Exports anesthesia-related equipment from companies like Air Liquide.
- Switzerland: Known for precision engineering, exports high-end components and systems.
10. Market Trends
- Current Global Trends:
- Integration & Connectivity: Seamless data flow to EMRs and centralized monitoring.
- Portability & Modularity: Growth in compact systems for outpatient and ambulatory centers.
- Rising Demand in Emerging Markets: Expanding healthcare infrastructure in Asia-Pacific and Latin America.
- New Technologies:
- Closed-Loop Anesthesia Delivery: Systems that automatically adjust anesthetic infusion and ventilation based on real-time patient feedback (BIS, hemodynamics).
- Advanced Gas Monitoring: Multi-gas Raman spectroscopy for faster, more accurate analysis.
- Touchscreen & Gesture Control: More intuitive user interfaces.
- Demand Drivers:
- Rising global surgical volumes.
- Aging populations requiring more surgical interventions.
- Emphasis on patient safety and operational efficiency in hospitals.
- Growth of day-case surgeries.
- Future Insights:
- Increased use of Artificial Intelligence (AI) for predictive alerts, decision support, and automated record-keeping.
- Enhanced Environmental Sustainability: Focus on ultra-low flow anesthesia and agents with lower global warming potential.
- Tele-anesthesia Support: Remote monitoring and guidance capabilities built into machines.
11. Training
Required Competency
Operators must be formally trained medical professionals (anesthesiologists, anesthetists, anesthesiology residents). Competency includes:
- Understanding the principles of anesthesia physics and physiology.
- Mastering the pre-use checklist.
- Proficiency in setting ventilation parameters, gas flows, and vaporizer settings.
- Ability to troubleshoot common alarms and failures (e.g., low pressure, apnea, O₂ sensor failure).
- Knowledge of emergency procedures (e.g., switching to backup ventilation).
Common User Errors
- Skipping or Rushing the Pre-Use Check: The root cause of many preventable incidents.
- Misinterpreting Alarms: Turning off an alarm without addressing the underlying cause.
- Incorrect Vaporizer Filling: Using the wrong agent or overfilling.
- Failure to Recognize Circuit Leaks/Disconnections.
- Inadequate Monitoring: Over-reliance on the machine without cross-checking with patient clinical signs.
Best-Practice Tips
- Always follow a pre-use checklist religiously.
- Never bypass safety features (e.g., oxygen monitor).
- Verify the oxygen sensor with room air at the start of each day.
- Have a self-inflating resuscitation bag (Ambu bag) immediately available as a backup ventilator.
- Stay with the machine during initial induction and stabilization of the patient.
12. FAQs
- Q: What is the single most important safety check before using the machine?
- A: Verifying that the oxygen analyzer is working and reads 21% in room air, and that you can achieve a 100% oxygen reading when flushing O₂.
- Q: How often should the CO₂ absorbent (soda lime) be changed?
- A: Change it when the color indicator changes (e.g., from pink to purple), or if it becomes excessively hard or crumbly. It’s standard practice to change it daily or between cases if exhausted.
- Q: Can I use the same machine for an adult and then a child?
- A: Yes, but you must change the breathing circuit to an appropriate pediatric circuit, ensure the ventilator settings are correctly adjusted for the child’s weight, and perform a full pre-use check.
- Q: What should I do if the machine alarms for “Low O₂ Concentration”?
- A: Immediately switch the patient to 100% oxygen via the integrated emergency O₂ flush button, ventilate with the backup Ambu bag if necessary, and then troubleshoot (check gas supplies, flowmeters, O₂ sensor calibration).
- Q: Are modern anesthesia machines MRI-safe?
- A: Standard machines are not. Specific MRI-compatible models are constructed from non-ferromagnetic materials and have specialized ventilators and monitors designed to function in the high-magnetic-field environment.
- Q: Why is the circle breathing system the most common?
- A: It conserves heat and humidity, reduces pollution, and significantly lowers the consumption of expensive anesthetic gases and oxygen, especially during low-flow anesthesia.
- Q: What is the purpose of the “O₂ Failure Protection Device”?
- A: It is a mechanical device that shuts off or dramatically reduces the flow of all other gases (like N₂O) if the oxygen supply pressure drops, preventing the delivery of a hypoxic gas mixture.
- Q: How long does the internal battery last?
- A: Typically 30 minutes to 3 hours depending on the model and load (running ventilator, monitors, screen). It is meant for short-term transport or power failure bridging, not prolonged use.
- Q: Can I use two different anesthetic agents in vaporizers on the same machine at the same time?
- A: Modern machines have vaporizer interlock systems that prevent more than one vaporizer from being turned on simultaneously to avoid accidental delivery of mixed agents.
- Q: Who is responsible for the routine maintenance of the machine?
- A: The clinical user is responsible for daily pre-use checks and cleaning. Biomedical engineering departments or the manufacturer’s service engineers are responsible for scheduled preventive maintenance, calibration, and repairs.
13. Conclusion
The modern anesthesia machine is a technological marvel, a cornerstone of patient safety in the operating room and beyond. It has evolved from a simple gas delivery apparatus into an intelligent, integrated workstation that supports anesthesiologists in providing precise, safe, and efficient care. Its complexity demands respect, thorough training, and meticulous adherence to safety protocols—most importantly, the pre-use checkout. Understanding its components, principles, regulations, and proper maintenance is essential for all clinicians who rely on it. As technology advances, these machines will become even more integrated, intuitive, and intelligent, further enhancing their role in safeguarding patients during some of medicine’s most critical moments.
14. References
- American Society of Anesthesiologists. (2020). Standards for Basic Anesthetic Monitoring.
- Dorsch, J. A., & Dorsch, S. E. (2020). Understanding Anesthesia Equipment (8th ed.). Wolters Kluwer.
- U.S. Food and Drug Administration. (FDA). Anesthesia Apparatus Checkout Recommendations.
- International Organization for Standardization. (2019). ISO 80601-2-13:2019 Medical electrical equipment — Part 2-13: Particular requirements for basic safety and essential performance of an anaesthetic workstation.
- Ehrenwerth, J., Eisenkraft, J., & Berry, J. (2020). Anesthesia Equipment: Principles and Applications (3rd ed.). Elsevier.
- World Health Organization. (WHO). Guidelines for Safe Surgery.
- Global Market Insights. (2023). Anesthesia Workstation Market Report.
- Manufacturer technical manuals and user guides (Dräger, GE HealthCare, Mindray).