1. Definition
What is an Anesthesia Cart?
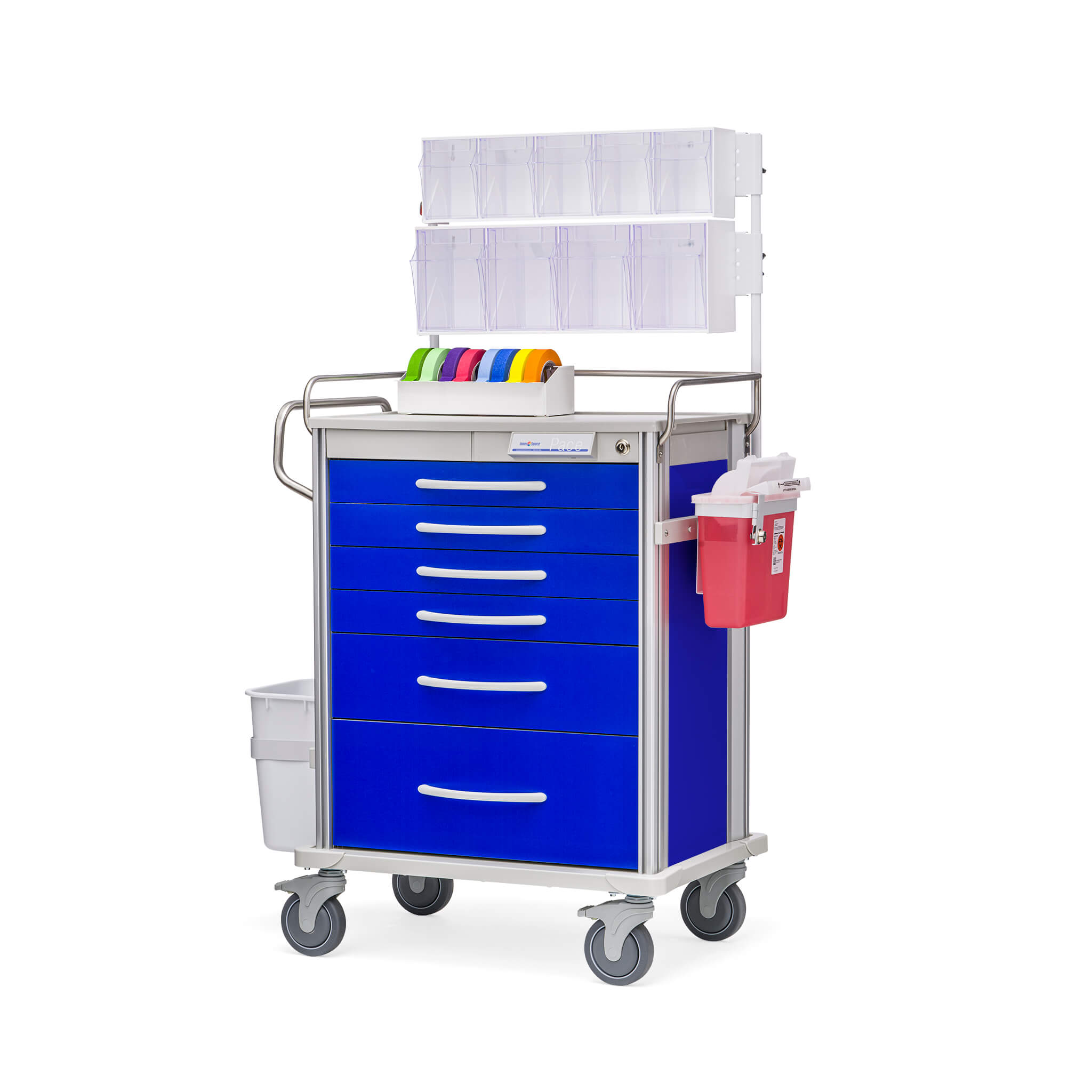
An anesthesia cart, often called an anesthesia trolley or anesthesia machine cart, is a mobile workstation specifically designed to store, organize, and transport the essential drugs, equipment, and supplies required for the safe administration of anesthesia. It is a critical piece of support equipment found in every operating theater and procedural area where anesthesia is delivered. Its primary function is to provide the anesthesia provider (Anesthesiologist or Nurse Anesthetist) with immediate, organized, and reliable access to everything needed before, during, and after an anesthetic procedure, thereby enhancing efficiency, safety, and readiness.
How it Works
The anesthesia cart itself is not a “working” medical device like a ventilator; rather, it is a sophisticated organizational system. It operates on the principle of systematic storage and ergonomic access. It is strategically stocked according to standardized protocols (often using color-coding and labeled sections) to allow the anesthesia professional to locate any item—from emergency drugs to airway equipment—quickly and intuitively, often in high-stress situations. Advanced models may integrate with facility inventory systems to track drug usage and automate restocking.
Key Components
A typical anesthesia cart consists of several core sections:
- Lockable Drug Drawers: The most critical component. Drawers are often segmented, lined, and labeled for controlled substances (with double-locks), emergency drugs (e.g., vasopressors, antiarrhythmics), and routine induction/maintenance agents. Many follow the “ABC” (Airway, Breathing, Circulation) or a similar acronym-based organization.
- Airway Management Compartment: A dedicated, often lift-out tray or section containing laryngoscope handles and blades (different sizes), endotracheal tubes, stylets, supraglottic airways (LMAs), oral/nasal airways, and suction catheters.
- IV Supplies and Fluid Section: Holds intravenous catheters, extension sets, stopcocks, tape, antiseptic swabs, and often bags of IV fluids like saline or lactated Ringer’s.
- Procedure Tray/Workspace: A flat, cleanable surface on top of the cart for preparing syringes, setting up equipment, and placing monitoring devices. Some have raised edges to prevent items from rolling off.
- Syringe and Needle Storage: Includes compartments for various sizes of syringes, needles, and sharps disposal containers.
- Accessory Hooks and Holders: For hanging blood pressure cuffs, oxygen masks, or IV bags.
- Mobility System: Features heavy-duty, lockable casters (wheels) that provide smooth movement and secure positioning. Brakes are essential.
- Emergency Equipment Storage: Some carts have dedicated, quickly accessible spots for difficult airway devices (e.g., video laryngoscope), defibrillator pads, or a self-inflating bag (Ambu bag).
2. Uses
Clinical Applications
The anesthesia cart is used in any clinical scenario involving anesthesia care:
- Pre-operative Preparation: For drawing up induction agents, opioids, muscle relaxants, and emergency drugs before patient arrival.
- Induction & Intubation: Immediate access to all airway equipment and drugs needed to render the patient unconscious and secure their airway.
- Maintenance of Anesthesia: Organized storage for additional syringes of maintenance drugs, IV fluids, and access supplies.
- Emergency Management: Critical for rapid response to intraoperative events like hypotension, bronchospasm, or cardiac arrest. The organized layout allows instant access to life-saving drugs like epinephrine, atropine, and vasopressors.
- Post-operative/Procedural Support: Holds drugs for reversal (e.g., naloxone, flumazenil), antiemetics, and analgesics.
Who Uses It
Primarily used by:
- Anesthesiologists
- Certified Registered Nurse Anesthetists (CRNAs)
- Anesthesia Assistants
- Operating Room Nurses (for restocking and assistance)
Departments/Settings
- Main Operating Rooms (ORs)
- Ambulatory Surgery Centers
- Labor & Delivery Suites (for epidural carts)
- Interventional Radiology/Cardiology Labs
- Endoscopy Suites
- Intensive Care Units (ICUs) (for procedural sedation)
- Emergency Departments (for trauma intubation and sedation)
- Dental Surgery Offices (providing general anesthesia)
3. Technical Specifications
Typical Specifications
- Dimensions: Height: 36″ – 48″ (91-122 cm); Width: 20″ – 30″ (51-76 cm); Depth: 16″ – 22″ (41-56 cm).
- Weight Capacity: The work surface and drawers are designed to hold 50-100 lbs (23-45 kg) of equipment.
- Drawer Configuration: 4 to 10 drawers of varying heights, often including a shallow top drawer for frequently used items.
- Casters: 4″ or 5″ (10-13 cm) diameter, with two locking brakes. Some have central locking pedals.
Variants & Sizes
- Standard Anesthesia Cart: The most common type with multiple drawers and a workspace.
- Anesthesia Crash Cart: A specialized variant pre-configured with advanced cardiac life support (ACLS) drugs and equipment for cardiac/respiratory emergencies, often with a built-in defibrillator.
- Epidural/Labor Analgesia Cart: Smaller, dedicated carts for regional anesthesia procedures, holding trays, sterile drapes, and specific nerve block needles.
- Procedural Sedation Cart: Used in non-OR settings, stocked for moderate sedation protocols.
- Wall-Mounted Anesthesia Stations: Fixed units used in space-constrained areas.
Materials & Features
- Materials: Constructed from high-grade, medical-grade stainless steel (304 or 316) for durability, corrosion resistance, and ease of cleaning. Some use powder-coated steel for cost-effectiveness.
- Features:
- Soft-Close Drawers: Prevent loud slamming and protect contents.
- Liner Trays: Removable, color-coded plastic trays for easy cleaning and organization.
- Electronic Locking: Audit trails for controlled substance access (e.g., PIN codes, fingerprint scanners).
- Integrated Power/Charging: Outlets and USB ports for charging devices like video laryngoscopes.
- Transparent Lids: On drawers for quick visual inventory without opening.
Models
Notable model series include:
- Omnicell Anesthesia Workstation
- Herman Miller Nemschoff Chatham Cart
- Medline Anesthesia Carts
- Waterloo Anesthesia Carts
- Lakeside Anesthesia Carts
4. Benefits & Risks
Advantages
- Enhanced Patient Safety: Reduces medication errors through standardized organization and labeling. Ensures emergency drugs are always present and accessible.
- Improved Efficiency: Saves critical time for the anesthesia provider by eliminating searches for equipment.
- Controlled Substance Security: Lockable, compliant storage for Schedule II-V drugs.
- Ergonomics: Reduces physical strain by bringing all necessary items to the point of care in an organized manner.
- Infection Control: Non-porous, cleanable surfaces reduce the risk of cross-contamination.
Limitations
- Requires Rigorous Restocking: Completely dependent on consistent human processes for restocking after each case.
- Can Become Cluttered: If not meticulously managed, it can become disorganized, negating its benefits.
- Mobility Hazards: If overloaded or on uneven floors, it can be a tipping hazard or difficult to maneuver.
- Fixed Configuration: Traditional carts may not adapt perfectly to highly specialized procedures without customization.
Safety Concerns & Warnings
- Never use a cart with malfunctioning or unlocked casters.
- Avoid overloading drawers or the top workspace.
- Ensure controlled substance drawers are locked at all times when not in immediate use.
- Regularly check and remove expired medications.
- Do not use damaged carts with sharp edges or broken drawers.
Contraindications
There are no medical contraindications for the cart itself. However, a non-stocked, disorganized, or poorly maintained cart is contraindicated for clinical use as it poses a direct risk to patient safety.
5. Regulation
The anesthesia cart, as a storage unit, is typically regulated as a Class I medical device in many jurisdictions because it poses minimal risk. However, if it has integrated electronic locking or inventory management systems, it may be classified as a higher class (e.g., Class II).
- FDA Class: Generally Class I (exempt from premarket notification, 510(k)), subject to general controls.
- EU MDR Class: Typically Class I under Rule 1.
- CDSCO Category: Usually classified under Class A (low risk) in India.
- PMDA Notes: In Japan, basic carts are regulated as Class I controlled medical devices.
- ISO/IEC Standards:
- ISO 13485: Quality Management Systems for Medical Devices (for manufacturers).
- IEC 60601-1: Safety standards for medical electrical equipment (if electrically powered).
- ISO 23908: Sharps injury protection standards (related to sharps containers on the cart).
6. Maintenance
Cleaning & Sterilization
- Daily/After Each Case: Wipe down all external surfaces, the worktop, drawer handles, and casters with a hospital-grade disinfectant wipe (e.g., bleach or quaternary ammonium compound). Non-lint cloths should be used.
- Weekly/Deep Clean: Remove all drawer liners and trays. Wash them with mild soap and water, then disinfect. Clean the interior of the drawers and the cart frame.
Reprocessing
The cart itself is non-critical and requires disinfection, not sterilization. Any sterile equipment stored within (airway devices, kits) must be reprocessed according to their own specific, validated sterilization protocols (e.g., steam autoclave, ethylene oxide).
Calibration
The cart requires no calibration. Any monitoring devices stored on it (e.g., a portable SpO2 probe) must be calibrated per manufacturer instructions.
Storage
- Store in a clean, dry environment within the operating room or anesthesia preparation area.
- Ensure brakes are engaged when stationary.
- Do not store in corrosive environments.
- Keep away from extreme heat sources.
7. Procurement Guide
How to Select the Device
- Assess Workflow: Observe how your anesthesia team works. Do they prefer many small drawers or fewer, larger ones?
- Consider Volume & Specialties: High-volume ORs may need larger carts. Cardiac or pediatric centers may need specialized configurations.
- Evaluate Space: Measure doorways and OR layouts to ensure the cart can move freely and fit in its designated spot.
- Prioritize Durability vs. Budget: Stainless steel is more durable but costly; coated steel is more affordable.
Quality Factors
- Smooth drawer operation (full-extension, ball-bearing slides).
- Quality of casters and braking mechanism.
- Sturdiness and stability (no wobbling).
- Ease of cleaning (seamless welds, non-porous surfaces).
- Quality of locks (both mechanical and electronic).
Certifications
Look for:
- FDA Registered Manufacturer (for US market).
- CE Marking (for EU market).
- ISO 13485 Certification of the manufacturer.
Compatibility
Ensure drawer liner sizes are standard or that the manufacturer offers a range of compatible organizational inserts. Check compatibility with existing controlled substance security systems if upgrading to electronic locks.
Typical Pricing Range
- Basic Model (Coated Steel): $800 – $2,000
- Standard Stainless Steel Model: $2,500 – $5,000
- Advanced Model (with electronic locks, charging): $5,000 – $10,000+
8. Top 10 Manufacturers (Worldwide)
- Omnicell (USA): Leader in medication management; known for advanced automated anesthesia workstations with inventory tracking.
- Herman Miller / Nemschoff (USA): Renowned for healthcare furniture, offering ergonomic and aesthetically designed carts.
- Medline (USA): Major healthcare supplier offering a wide range of durable, cost-effective anesthesia and procedure carts.
- InterMetro (USA): Known for wire shelving and carts; offers robust, functional anesthesia carts used in many hospitals.
- Waterloo Healthcare (USA): Specializes in hospital-grade stainless steel carts, including anesthesia and emergency carts.
- Lakeside Manufacturing (USA): Produces a wide array of stainless steel medical carts with high durability.
- Armstrong Medical (USA/UK): Known for its “Tuffcart” series and specialized resuscitation carts, including anesthesia models.
- Anetic Aid (UK): UK-based manufacturer of medical equipment, including a range of procedure and anesthesia trolleys.
- Alvo Medical (Poland): European manufacturer producing a wide range of medical trolleys and carts, including anesthesia.
- Hawksmed (UK/Singapore): Provides medical trolleys and carts, with a focus on the UK and Asian markets.
9. Top 10 Exporting Countries (Latest Year – Based on HS Code 940290: Medical Furniture)
Rankings are illustrative based on global medical furniture export trends.
- China: The world’s largest exporter, producing a vast range of carts from economical to high-quality models.
- Germany: Exports high-end, precision-engineered medical carts and trolleys.
- United States: Major exporter of advanced, technology-integrated carts and workstations.
- Italy: Known for design-oriented medical furniture, including anesthesia carts.
- Poland: A growing hub in the EU for cost-effective, quality medical cart manufacturing.
- Mexico: Key exporter to the North American market under trade agreements.
- United Kingdom: Exports specialized carts from manufacturers like Anetic Aid.
- Canada: Exports to the US and other markets, often with a focus on durable goods.
- France: Exports medical equipment including anesthesia-related furniture.
- Netherlands: A major EU trading hub for medical devices and equipment.
10. Market Trends
Current Global Trends
- Integration with Hospital Information Systems (HIS): Carts are becoming nodes in the hospital network for real-time inventory tracking and automated reordering.
- Focus on Ergonomics and Clinician Well-being: Designs that reduce bending, reaching, and physical strain.
- Rise of Single-Patient Use Carts: In some settings, especially post-COVID-19, to minimize cross-contamination risks.
New Technologies
- RFID (Radio-Frequency Identification): For automated tracking of every item in the cart.
- Biometric/Smart Card Locks: For enhanced security and detailed audit trails of controlled substance access.
- Modular Design: Allowing departments to customize drawer configurations easily.
Demand Drivers
- Rising global surgical volumes.
- Stringent medication safety regulations and accreditation standards (e.g., Joint Commission).
- Growth of ambulatory surgery centers (ASCs).
- Focus on reducing preventable medical errors.
Future Insights
The anesthesia cart will evolve from a passive storage unit to an active, intelligent perioperative assistant. Expect deeper AI integration for predictive restocking, barcode scanning at point-of-use, and seamless data integration with the anesthesia machine and electronic medical record (EMR).
11. Training
Required Competency
All users must be trained on:
- The specific organizational layout and color-coding system of the cart.
- Proper procedures for accessing and documenting controlled substances.
- Safe maneuvering and locking of the cart.
- The restocking protocol and how to check for expired items.
Common User Errors
- Failing to Restock Immediately: Leaving the cart depleted for the next case.
- “Borrowing” from a Neighbor’s Cart: Disrupts inventory and can cause critical shortages.
- Ignoring Expiry Dates: Not rotating stock leads to use of expired drugs.
- Overloading Drawers: Causes jams and makes it difficult to find items.
- Not Locking Controlled Substance Drawers: A major regulatory and safety violation.
Best-Practice Tips
- Perform a “Cart Check” at the start of every shift: Verify all critical drugs and equipment are present and within expiry.
- Follow the “One-Touch” Rule: Restock any item as soon as it is taken, if possible, or immediately after the case concludes.
- Keep the Workspace Clear: Use it only for immediate case preparation, not for long-term storage.
- Engage Brakes Always: When the cart is not being actively moved.
12. FAQs
1. How often should an anesthesia cart be restocked?
Ideally, it should be restocked immediately after each case and undergo a full check at the start of each day or shift.
2. Who is responsible for restocking the anesthesia cart?
This varies by facility. It is often a shared responsibility between anesthesia providers, OR pharmacy technicians, and/or dedicated anesthesia assistants or nurses, following a clear protocol.
3. Can we customize the drawer layouts?
Yes, most manufacturers offer customizable liner trays and dividers. It’s highly recommended to customize based on your department’s specific workflow and drug preferences.
4. What is the difference between an anesthesia cart and a crash cart?
An anesthesia cart is tailored for routine and emergency anesthesia care. A crash cart is specifically configured for cardiopulmonary resuscitation (CPL) emergencies, containing ACLS drugs, a defibrillator, and cardiac arrest-specific equipment.
5. How do we manage controlled substances on the cart?
They must be kept in a double-locked compartment (a locked drawer within a locked cart). Access must be logged manually or electronically per DEA (or local regulatory) and hospital policy.
6. What cleaning agent is safe for the stainless steel surface?
Use hospital-grade disinfectant wipes. Avoid abrasive pads or harsh chemicals like chloride-based bleaches on stainless steel for prolonged periods, as they can cause pitting and corrosion.
7. How long does a typical anesthesia cart last?
With proper maintenance, a high-quality stainless-steel anesthesia cart can last 10-15 years or more in a hospital environment.
8. Are there guidelines for how to organize the cart?
While no single universal standard exists, many institutions follow guidelines from bodies like the Anesthesia Patient Safety Foundation (APSF) or use recognized principles like “METRIC” (Machine, Equipment, Drugs, IVs, Towels, Clean-up) or “SOAPME” for pediatric carts.
9. Can the cart be connected to power for charging devices?
Many modern carts have integrated power strips with outlets and USB ports. Ensure the cord is managed safely to avoid a tripping hazard.
10. What should be done if a controlled substance is missing from the cart?
Follow your institution’s immediate incident reporting protocol. This typically involves notifying the pharmacy, the supervising anesthesiologist, and security, and conducting an investigation.
13. Conclusion
The anesthesia cart is far more than a simple trolley; it is the indispensable nerve center of anesthesia practice. Its thoughtful design and meticulous organization are foundational to patient safety, clinician efficiency, and operational readiness. From routine surgeries to life-threatening emergencies, a well-stocked, well-maintained anesthesia cart ensures that the right tool and the right drug are available at the right moment. As technology advances, its role is expanding from passive storage to an intelligent partner in perioperative care. Investing in the right cart and, more importantly, instituting robust processes for its use and maintenance, is a critical commitment to excellence in any anesthesia delivery setting.
14. References
- American Society of Anesthesiologists (ASA). (2023). Standards and Practice Parameters.
- Anesthesia Patient Safety Foundation (APSF). (2020). Recommendations for Pre-Anesthesia Checkout Procedures.
- Food and Drug Administration (FDA). (2023). Code of Federal Regulations, Title 21, Part 880 – General Hospital and Personal Use Devices.
- International Organization for Standardization (ISO). ISO 13485:2016. Medical devices — Quality management systems.
- Joint Commission, The. (2024). Medication Management (MM) Standards for Hospitals.
- Roth, D., & Lubarsky, D. A. (2021). “The Anesthesia Workstation: A Primer on Cart to Machine.” Anesthesiology Clinics.
- (Note: Manufacturer websites and technical catalogs from Omnicell, Medline, Waterloo, etc., were also consulted for specifications and features.)