1. Definition
What is a Circular Surgical Stapler?
A circular surgical stapler is a specialized, mechanically operated medical device designed to create a secure, leak-proof connection between two hollow structures or organs in the body, most commonly after a segment has been removed. Think of it as a highly sophisticated hole punch and stapler that works in a single, precise action. Its primary function is to perform an anastomosis—the surgical joining of two separate tubular structures, such as intestines, the esophagus, or the stomach.
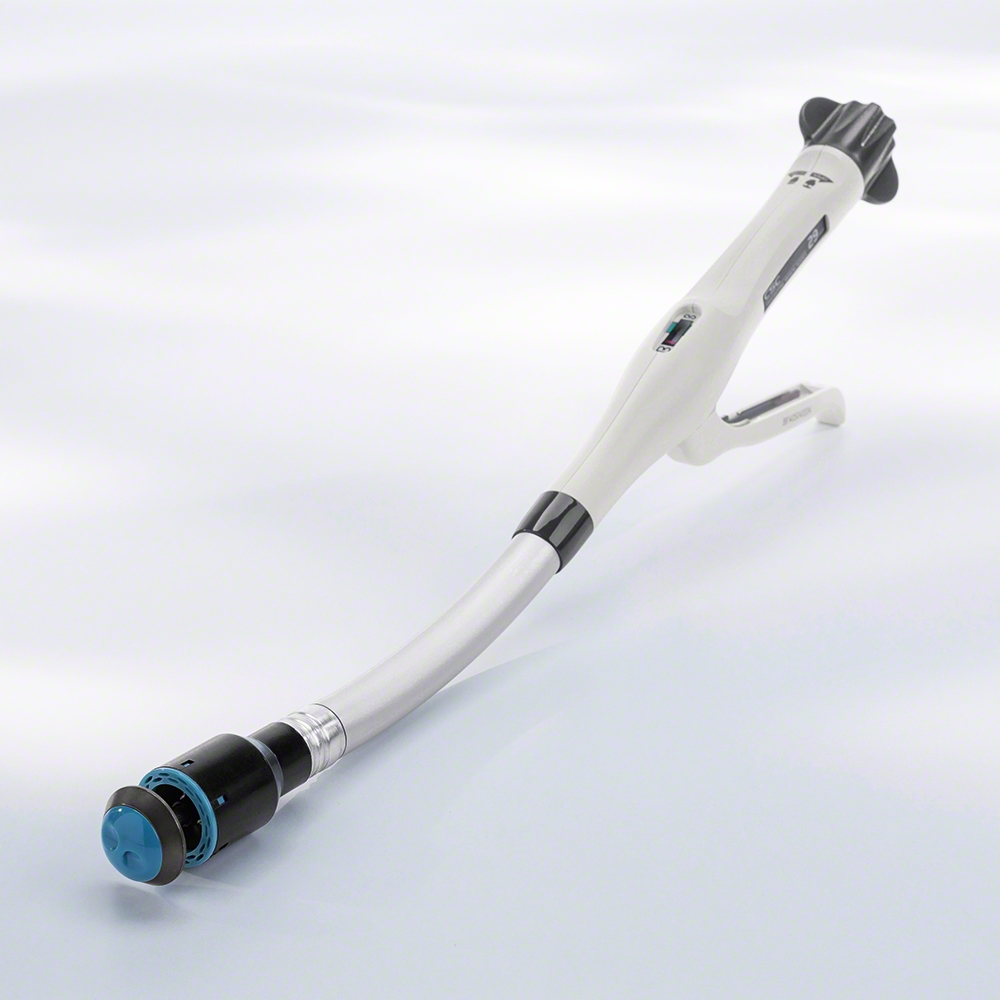
This device has revolutionized gastrointestinal, thoracic, and bariatric surgery by enabling complex reconstructions that are faster, more consistent, and often lead to better patient outcomes compared to traditional hand-sewn suturing techniques.
How it Works
The principle of a circular stapler is elegantly simple, involving a “B” formation of staples.
- Positioning: The device is inserted, often through a natural orifice or a surgical opening. The two ends of the hollow organs to be joined are prepared. One end is secured with a “purse-string suture” around the anvil (a cone-shaped component), and the other is secured around the stapler body.
- Approximation: The surgeon carefully brings the anvil and the stapler body together by turning a knob on the handle. This approximation compresses the two tissue sections between the anvil and the stapler head.
- Firing: The surgeon squeezes the firing lever. This single action drives a circular array of tiny B-shaped surgical staples through both layers of tissue, molding them against the anvil to form the “B” shape. Simultaneously, a circular blade within the stapler head advances, cutting the excess tissue inside the newly formed staple line, creating a clean, open lumen (channel).
- Removal: The anvil is slightly separated, and the entire device is gently removed.
The result is a patent, circular anastomosis that is both hemostatic (prevents bleeding) and airtight/leak-proof.
Key Components
- Stapler Body/Handle: The main unit containing the firing mechanism and approximation knob. It houses the staple cartridge.
- Shaft: A long, narrow tube that connects the handle to the stapler head, allowing for deep access within the body.
- Stapler Head (Cartridge): Contains a circular array of pre-loaded staples (typically between 21 and 33) and the circular blade.
- Anvil: The cone-shaped component that mates with the stapler head. It is responsible for forming the B-shaped staples as they are fired. It is either detachable or permanently attached, depending on the model.
- Anvil Shaft: The post that connects the anvil to the stapler head.
- Approximation Knob: Located on the handle, this knob controls the opening and closing of the anvil, allowing the surgeon to adjust the tissue gap.
- Firing Lever/Safety: The trigger that deploys the staples and blade. It often has a safety mechanism to prevent accidental firing.
- Indicator/Tissue Gap Scale: A visual or numerical indicator that shows the distance between the anvil and the stapler head, helping the surgeon achieve the optimal compression for the tissue type.
2. Uses
Clinical Applications
Circular staplers are indispensable in a wide range of surgical procedures, primarily within:
- Colorectal Surgery:
- Low Anterior Resection (LAR): For rectal cancer, reconnecting the colon to the rectum after tumor removal.
- Colectomy: Reconnecting the colon after removing a diseased segment (e.g., due to cancer or diverticulitis).
- Ileal Pouch-Anal Anastomosis (IPAA): In ulcerative colitis patients, creating a new “pouch” from the small intestine and connecting it to the anus.
- Upper GI Surgery:
- Esophagectomy: Connecting the stomach or a colon graft to the esophagus after esophageal removal.
- Gastrectomy: Reconnecting the stomach to the small intestine (gastrojejunostomy) after partial or total stomach removal.
- Roux-en-Y Gastric Bypass: A key tool in bariatric surgery for creating the small stomach pouch and the intestinal bypass connections.
- Thoracic Surgery: For bronchial or tracheal anastomoses, though less common than GI applications.
- Bariatric Surgery: As mentioned above, for gastric bypass procedures.
Who Uses It
- Surgeons: Primarily Colorectal Surgeons, General Surgeons, Surgical Oncologists, Bariatric Surgeons, and Thoracic Surgeons.
- Surgical Assistants/Scrub Nurses: Responsible for preparing, loading, and handing the device to the surgeon in a sterile manner, ensuring it is correctly assembled and ready for use.
Departments/Settings
- Operating Rooms (ORs) in hospitals and major surgical centers.
- Specialized Surgical Suites in ambulatory surgery centers (ASCs) for certain procedures.
3. Technical Specs
Typical Specifications
- Diameter: The internal diameter of the anastomosis is the most critical spec, typically ranging from 21 mm to 33 mm.
- Shaft Length: Varies from short (for accessible areas) to long (for deep pelvic surgery), e.g., 45 mm to 55 mm.
- Staple Height (or Leg Length): The height of the formed staple, designed for specific tissue thickness (e.g., 1.0 mm, 1.5 mm, 2.0 mm). Modern staplers often feature adaptive or variable height staples.
- Number of Staple Rows: Most circular staplers have two staggered rows of staples for enhanced security.
Variants & Sizes
- Reusable vs. Disposable: Reusable staplers (less common now) require cleaning and reloading. Single-use disposable staplers are the standard due to guaranteed sterility and performance.
- Anvil Type: Detachable anvils offer greater flexibility, especially in minimally invasive surgery. Permanent anvils are fixed to the shaft.
- Shaft Design: Curved shafts or rotatable heads are available to improve access and visibility in challenging anatomies.
Materials & Features
- Materials: Primarily medical-grade plastics (e.g., polycarbonate) and stainless steel or titanium for the staples and anvil.
- Key Features:
- Tri-Staple Technology: Uses three staggered staple rows of different heights to create a stronger, more hemostatic anastomosis.
- Powered Stapling: Electric-powered staplers offer consistent firing force and can reduce surgeon fatigue.
- Tissue Compression Indicators: Visual scales that guide the surgeon on optimal compression time to prevent tissue damage.
- Knit-Line™ or Gore Seam Guard: Reinforcing materials that can be used with the stapler to buttress the staple line, reducing the risk of leakage or bleeding in fragile tissues.
Models
- Medtronic: DST Series™ EEA™ (EEA = End-to-End Anastomosis), Signia™ (Powered).
- Johnson & Johnson (Ethicon): CDH / ECS Contour™ Curved Cutter, ECHELON CIRCULAR™ Powered Stapler with Gripping Surface Technology (GST).
- Intuitive Surgical: EndoWrist® Stapler 45 (for use with the da Vinci robotic system).
4. Benefits & Risks
Advantages
- Reduced Operative Time: Significantly faster than manual suturing.
- Consistency and Reliability: Creates a uniform, standardized anastomosis.
- Reduced Leak Rates: When used correctly, can offer lower anastomotic leak rates compared to hand-sewn techniques in certain procedures.
- Improved Hemostasis: The staple lines provide excellent hemostasis.
- Facilitates Minimally Invasive Surgery: Enables complex anastomoses through small laparoscopic or robotic ports.
Limitations
- Cost: Disposable staplers are expensive consumables.
- Learning Curve: Requires specific training to use safely and effectively.
- Rigidity: The device is relatively rigid, which can be challenging in very confined spaces like a narrow male pelvis.
- Tissue Mismatch: Can be difficult to use when joining two structures of drastically different diameters.
Safety Concerns & Warnings
- Anastomotic Leak: The most serious complication, which can lead to sepsis.
- Bleeding: From the staple line.
- Stricture: Narrowing of the anastomosis over time.
- Tissue Trauma: From over-compression or improper handling.
- Device Malfunction: Staple line malformation, failure to fire, or failure of the knife to cut.
Contraindications
- Use in tissue that is necrotic, ischemic, or severely inflamed/edematous.
- When the tissue thickness is outside the range specified for the stapler.
- In patients with known hypersensitivity to the staple materials (e.g., nickel, titanium).
5. Regulation
Circular staplers are high-risk devices due to their critical function and are heavily regulated worldwide.
- FDA Class: Class II (Special Controls). Requires a 510(k) premarket notification to demonstrate substantial equivalence to a predicate device.
- EU MDR Class: Class IIb (Rule 10 – Surgically invasive devices intended for use in direct contact with the heart, central circulatory system or central nervous system, or devices intended specifically to control, diagnose, monitor or correct a defect of the heart or central circulatory system…). In practice, anastomosis devices fall under high-risk classification.
- CDSCO Category (India): Class C (Moderate to High Risk), equivalent to a US FDA Class II/III or EU MDR Class IIb/III.
- PMDA (Japan): Classified as “Controlled Medical Devices” (Class II). Requires certification from a Registered Certified Body (RCB).
- ISO/IEC Standards:
- ISO 13485: Quality Management Systems for Medical Devices.
- ISO 15223-1: Symbols to be used on labels and documentation.
- IEC 60601-1: Safety for electrical medical devices (for powered staplers).
6. Maintenance
This section primarily applies to reusable or reposable devices. Single-use devices are disposed of after one procedure.
- Cleaning & Sterilization: For reusable components, strict adherence to the manufacturer’s Instructions for Use (IFU) for cleaning and sterilization (typically steam autoclaving) is mandatory to prevent biofilm formation and ensure sterility.
- Reprocessing: Single-use devices are not to be reprocessed or reused. Re-posable devices (where only the stapler cartridge is replaced) require specific steps for disassembly and reloading as per IFU.
- Calibration: Checked for function and calibration before each use (e.g., ensuring the anvil clicks into place, the approximation knob moves smoothly).
- Storage: Store in a cool, dry place, protected from light and physical damage. Avoid stacking heavy objects on the packages. Observe “use-by” dates on single-use devices.
7. Procurement Guide
How to Select the Device
Consider:
- Procedure Type: What surgeries will it be used for? (e.g., colorectal vs. bariatric).
- Surgical Approach: Open, laparoscopic, or robotic? This dictates shaft length and anvil type.
- Tissue Characteristics: Patient population and typical tissue thickness.
- Hospital Budget: Balance between cost and desired features (e.g., powered vs. manual).
Quality Factors
- Reliability and consistency of staple formation.
- Ease of use and ergonomics for the surgeon.
- Reputation and technical support of the manufacturer.
- Clinical data supporting safety and performance.
Certifications
Ensure the device has the necessary regulatory marks for your region: CE Mark (for EU), FDA Clearance (for USA), and other local certifications.
Compatibility
- Ensure compatibility with existing stapler reloads if using a re-posable system.
- For robotic surgery, the stapler must be compatible with the specific robotic platform.
Typical Pricing Range
Circular staplers are high-cost items. Single-use disposable staplers can range from $400 to over $1,200 per unit, depending on the technology, features, and geographic market.
8. Top 10 Manufacturers (Worldwide)
- Medtronic plc (Ireland/USA): A global leader in medical technology. Notable for the DST Series™ and Signia™ Powered Staplers.
- Johnson & Johnson (Ethicon Inc.) (USA): A historic and dominant player. Notable for the ECHELON CIRCULAR™ and Contour Curved Cutter staplers.
- Intuitive Surgical, Inc. (USA): The pioneer in robotic-assisted surgery. Its EndoWrist® Staplers are designed exclusively for the da Vinci system.
- B. Braun Melsungen AG (Germany): A major European player offering a range of surgical staplers.
- 3M (KCI) (USA): Known for its skin closure products, it also has a presence in internal stapling.
- Smith & Nephew plc (UK): A global medical technology business focused on orthopedics and advanced wound management, with a stake in surgical devices.
- CONMED Corporation (USA): Offers a portfolio of surgical devices, including staplers.
- Becton, Dickinson and Company (BD) (USA): While stronger in diagnostics, BD has a presence in surgical instrumentation.
- Welfare Medical Ltd. (UK): A specialist manufacturer and supplier of surgical staplers and accessories.
- Purple Surgical (UK): An independent company specializing in the design and manufacture of surgical stapling devices.
9. Top 10 Exporting Countries (Latest Year)
Based on analysis of trade data for HS Code 901890 – Instruments and appliances used in medical sciences.
- United States: The dominant exporter, home to J&J and Medtronic’s core operations.
- Ireland: A major hub for Medtronic’s manufacturing and global exports.
- Germany: A traditional powerhouse in medical device manufacturing (B. Braun).
- Mexico: A key manufacturing location for the North American market.
- China: A rapidly growing manufacturer and exporter of medical devices, including staplers.
- United Kingdom: Home to several specialized manufacturers like Purple Surgical and Welfare Medical.
- Japan: A strong domestic market with high-quality manufacturing standards.
- France: Hosts significant manufacturing facilities for global corporations.
- Switzerland: Known for precision engineering in medical devices.
- Singapore: A strategic Asian hub for medical technology manufacturing and distribution.
10. Market Trends
- Current Global Trends: The market is experiencing steady growth driven by the rising prevalence of cancers (colorectal, gastric) and obesity, and the global shift towards minimally invasive surgeries (MIS).
- New Technologies:
- Widespread Adoption of Powered Staplers: Reducing variability in firing and surgeon fatigue.
- Robotic Integration: Staplers designed specifically for robotic platforms are a key growth area.
- Smart Staplers with Sensors: Emerging devices that provide real-time feedback on tissue compression and perfusion to optimize firing decisions.
- Advanced Bio-materials: The development of new staple lines and buttressing materials that are absorbable and promote healing.
- Demand Drivers: Aging population, rising surgical volumes, technological advancements, and patient demand for less invasive procedures.
- Future Insights: The future lies in data-driven, adaptive stapling. Expect staplers that integrate with hospital data systems, use AI to recommend settings based on patient anatomy, and provide even greater levels of safety and predictability.
11. Training
Required Competency
Proficiency requires a combination of didactic education, simulation-based training, and supervised clinical practice. Surgeons must understand the principles of anastomosis, device mechanics, and complication management.
Common User Errors
- Incorrect Purse-String Suture: Leading to tissue slippage and leaks.
- Over-compression of Tissue: Holding the tissue under high compression for too long, causing ischemia and necrosis.
- Firing over a Previous Staple Line: A significant risk for failure.
- Using the Wrong Staple Height: For the given tissue thickness.
- Excessive Force during Firing or Removal: Causing tissue tear.
Best-Practice Tips
- Know your IFU: Always review the manufacturer’s instructions for the specific device model.
- Choose the Right Size: Do not oversize or undersize the stapler; match it to the tissue.
- Check the “Doughnuts”: After firing, inspect the two rings of excised tissue to ensure they are complete and circular. An incomplete doughnut may indicate an incomplete anastomosis.
- Perform a Leak Test: Intraoperative testing with air or dye insufflation is a critical safety step.
- Ensure Good Blood Supply: Anastomose only well-perfused, healthy tissue.
12. FAQs
- What’s the difference between a linear and a circular stapler?
- Linear staplers cut and seal tissue in a straight line (e.g., for removing a lung lobe or transecting the stomach). Circular staplers create a circular connection between two hollow structures.
- Are the staples left inside the body?
- Yes. Surgical staples are made of biocompatible materials (titanium or absorbable polymers) and are designed to remain in the body permanently. They do not set off airport metal detectors.
- Can a circular stapler be used in laparoscopic surgery?
- Absolutely. Laparoscopic-specific circular staplers with long, narrow shafts and detachable anvils are commonly used in minimally invasive procedures.
- What happens if the stapler misfires?
- The surgeon must be prepared for this. The standard protocol is to carefully remove the device, assess the situation, and either refire a new stapler or convert to a hand-sewn anastomosis.
- How long does it take for the staples to heal?
- The staples hold the tissues in place while the body’s natural healing process joins them together. The initial healing takes 1-2 weeks, but full tissue remodeling takes months.
- What is an anastomotic leak and how common is it?
- It is a leak of intestinal contents from the anastomosis. It is a serious complication. Rates vary by procedure and patient factors but generally range from 1% to 10%. Proper stapler use is key to minimizing this risk.
- Why are there so many different sizes?
- Different anatomies and procedures require different lumen diameters. Using a size that is too small can lead to stricture, while one that is too large may not fit the anatomy or could cause tissue tearing.
- Is a stapled anastomosis better than a hand-sewn one?
- “Better” is context-dependent. For many procedures, large clinical studies show they are equivalent. The choice often depends on the surgeon’s experience, the specific procedure, and patient anatomy. Staplers offer speed and standardization, while hand-sewing offers ultimate flexibility.
13. Conclusion
The circular surgical stapler is a cornerstone of modern surgery, enabling complex reconstructions with remarkable efficiency and consistency. Its development represents a significant advancement in patient care, contributing to reduced operative times and improved outcomes in gastrointestinal, thoracic, and bariatric procedures. However, it is not a substitute for surgical skill and judgment. Its safe and effective use hinges on a thorough understanding of its principles, meticulous technique, rigorous training, and unwavering adherence to safety protocols. As technology evolves, the integration of power, data, and intelligence promises to make this already vital tool even more precise and safe in the years to come.
14. References
- Medtronic. (2023). DST Series™ EEA™ Circular Stapler Instructions for Use.
- Ethicon (Johnson & Johnson). (2023). ECHELON CIRCULAR™ Powered Stapler Technical Brochure.
- Cirocchi, R., et al. (2020). “Linear versus circular stapler anastomosis in laparoscopic colorectal surgery: a systematic review and meta-analysis.” Langenbeck’s Archives of Surgery.
- U.S. Food and Drug Administration (FDA). (2022). Classify Your Medical Device.
- European Commission. (2017). Regulation (EU) 2017/745 on medical devices (MDR).
- Central Drugs Standard Control Organization (CDSCO). (2020). Medical Device Rules.
- Grand View Research. (2023). Surgical Stapling Devices Market Size, Share & Trends Analysis Report.