1. Definition
What is an AED?
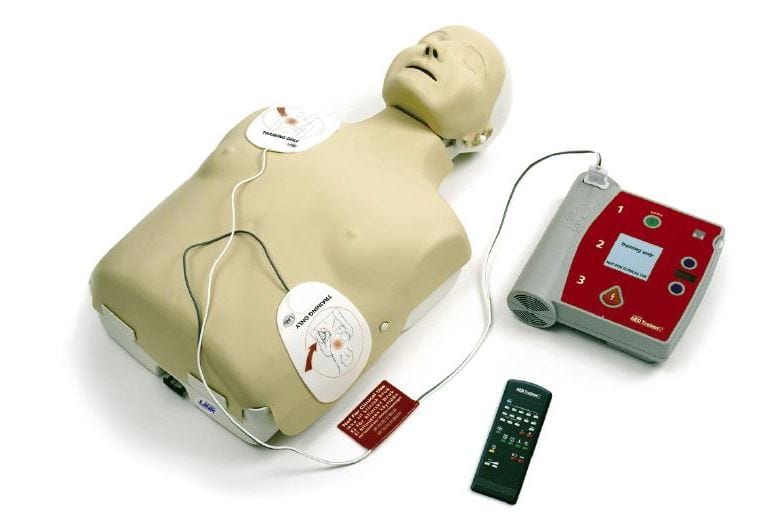
An Automated External Defibrillator (AED) is a portable, electronic medical device designed to diagnose and treat sudden cardiac arrest (SCA) by delivering an electric shock (defibrillation) to the heart. Its primary function is to restore a normal heart rhythm in a person experiencing a life-threatening arrhythmia, specifically ventricular fibrillation (VF) or pulseless ventricular tachycardia (VT). AEDs are intentionally built for simplicity, enabling use by laypersons with minimal training, as well as healthcare professionals.
How It Works
The AED works on a simple principle: analyze, advise, shock.
- Analysis: Once the adhesive electrode pads are placed on the patient’s bare chest, the AED’s built-in computer analyzes the heart’s electrical rhythm. It uses sophisticated algorithms to determine if the rhythm is “shockable” (VF or pulseless VT) or “non-shockable” (e.g., asystole or normal rhythm).
- Advisement: The device provides clear, step-by-step voice (and often visual) prompts to guide the rescuer. It will only advise a shock if one is medically necessary.
- Defibrillation: If a shock is advised, the device instructs the rescuer to stand clear and press the shock button. The AED then delivers a controlled electric current through the pads to the heart. This shock aims to depolarize the entire heart muscle simultaneously, stopping the chaotic rhythm and allowing the heart’s natural pacemaker to re-establish an effective rhythm.
Key Components
- Main Unit/CPU: The “brain” containing the battery, capacitor, and microprocessor that controls analysis and shock delivery.
- Electrode Pads: Adhesive pads placed on the patient’s chest. They have two functions: to sense the heart’s rhythm and to deliver the shock. They are often designed for specific patient sizes (adult vs. pediatric).
- Battery: Provides power for analysis and charges the capacitor to deliver the shock. Typically long-life lithium batteries, either non-rechargeable or rechargeable.
- Capacitor: Stores the electrical energy from the battery and releases it rapidly to generate the defibrillating shock.
- Display Screen & Voice Prompts: Provides visual and audio instructions to guide the user through the entire process.
- Carry Case/Storage Unit: Protects the device and often contains accessory kits (razor, towel, scissors).
2. Uses
Clinical Applications
The sole clinical application of an AED is the emergency treatment of sudden cardiac arrest (SCA) caused by shockable heart rhythms. Its use is a critical link in the Chain of Survival, specifically the link between early CPR and early defibrillation. For every minute that defibrillation is delayed, the chance of survival decreases by 7-10%.
Who Uses It?
- Lay Rescuers: Bystanders, family members, workplace colleagues, flight attendants, security personnel, and teachers. Public Access Defibrillation (PAD) programs rely on laypersons.
- First Responders: Police officers, firefighters, and emergency medical technicians (EMTs).
- Healthcare Professionals: Nurses, doctors, paramedics, and clinical staff in all hospital departments and outpatient clinics.
Departments/Settings
AEDs are ubiquitous due to the unpredictable nature of SCA.
- Public Settings: Airports, shopping malls, sports stadiums, gyms, schools, offices, and community centers (Public Access Defibrillators).
- Pre-hospital/Emergency Medical Services (EMS): Ambulances, fire engines, police vehicles.
- Hospital-wide: Beyond just the ER and ICU, they are found in hallways, outpatient clinics, dialysis units, radiology departments, and cafeterias for rapid response.
- Specialized Clinics: Dental offices, physiotherapy clinics, and cardiac rehabilitation centers.
3. Technical Specifications
Typical Specifications
- Energy Levels: Deliver biphasic truncated exponential waveform shocks. Typical escalating energies: 150J, 200J, then 200J+ for adults. Pediatric doses are typically 50-75J, often delivered via child pads or an energy attenuator.
- Analysis Time: Typically 5-15 seconds.
- Charge Time: Time to ready a shock after advice: Usually <10 seconds.
- Battery Life: Standby life of 2-5 years (for non-rechargeable lithium packs) or 300+ shocks/recharges.
- IP Rating: Often IP55 or higher for dust and water resistance.
- Operating Temperature: 0°C to 50°C (32°F to 122°F) for most models.
- Weight: 1.5 – 4.5 kg (3.3 – 10 lbs), including battery.
Variants & Sizes
- Fully Automated vs. Semi-Automated: Fully automated AEDs will deliver a shock automatically if advised, while semi-automatic models require the user to press a shock button when prompted.
- Professional vs. Public Access: Professional models may have more features (e.g., ECG display, manual override), while PAD models are designed for ultimate simplicity.
- Ruggedized Models: Designed for harsh environments (fire, military, industrial) with higher IP ratings and durable casings.
Materials & Features
- Construction: High-impact plastic (e.g., ABS, polycarbonate) casings.
- Key Features:
- Self-Testing: Daily, weekly, and monthly self-checks of battery, pads, and internal circuitry.
- CPR Feedback: Metronomes and real-time feedback on compression rate and depth (via pads with accelerometers).
- Wi-Fi/Connectivity: For remote monitoring of device status and incident data download.
- Rescue Guidance: Video screens demonstrating CPR and pad placement.
Notable Models
- Philips HeartStart FRx / OnSite: Industry stalwarts known for durability.
- ZOLL AED 3 / AED Plus: Feature Real CPR Help® for compression feedback.
- Defibtech Lifeline / Reviver: Known for clear voice prompts and simplicity.
- Cardiac Science Powerheart / G5: Feature robust self-testing and RescueReady® technology.
- Stryker (Physio-Control) LIFEPAK CR2: Offers intuitive user interface and connected capabilities.
4. Benefits & Risks
Advantages
- Dramatically Increases Survival Rates: Early defibrillation is the single most important factor in surviving SCA.
- Ease of Use: Designed for use by untrained individuals with clear, calm voice instructions.
- Safety: Sophisticated analysis ensures a shock is only delivered when needed. Prompts users to stand clear.
- Portability: Lightweight and instantly accessible in an emergency.
- Automatic Maintenance: Self-testing ensures readiness without daily manual checks.
Limitations
- Rhythm-Specific: Only effective for shockable rhythms (VF/pulseless VT). Ineffective for asystole (flatline) or PEA (pulseless electrical activity).
- Requires CPR: An AED is an adjunct to, not a replacement for, high-quality CPR.
- Environmental Interference: Can be affected by strong electromagnetic interference or excessive patient movement.
Safety Concerns & Warnings
- Water: Ensure the victim’s chest is dry before pad placement. Move them from standing water if possible.
- Flammable Environments: Do not use near flammable gases or materials. The spark from a shock is a theoretical risk.
- Pacemakers/ICDs: Place pads at least 1 inch away from an implanted device. It is safe to use an AED on someone with an implant.
- Medication Patches: Remove any medication patches (e.g., nitroglycerin) from the chest area and wipe clean before placing pads.
- Clearing the Patient: Always loudly state “STAND CLEAR” and visually ensure no one is touching the patient before analyzing or shocking.
Contraindications
- A Conscious, Responsive Patient with a Pulse: An AED should never be used on a person who is conscious and has a palpable pulse.
- A Patient with a ‘Do Not Resuscitate’ (DNR) Order: Respect legal and ethical advanced directives.
5. Regulation
AEDs are critical life-saving devices and are stringently regulated worldwide.
- FDA Class: Class III (US FDA). Considered high-risk, requiring Premarket Approval (PMA).
- EU MDR Class: Class III under Rule 9 of Annex VIII of the EU MDR.
- CDSCO Category: Class C (High Risk) as per the Medical Device Rules, 2017 in India.
- PMDA (Japan): Regulated as Class III specified controlled medical devices, requiring certification from Registered Certification Bodies (RCBs).
- ISO/IEC Standards:
- ISO 13485: Quality Management Systems for Medical Devices.
- ISO 60601-1 & -2: General safety & essential performance, with particular standards for defibrillators.
- ISO 14708-3: For Active Implantable Medical Devices (relevant for compatibility testing with pacemakers).
- IEC 62304: Medical device software lifecycle processes.
6. Maintenance
Proper maintenance is non-negotiable for device readiness.
- Cleaning & Sterilization: Wipe the exterior with a damp cloth and mild detergent. Do not immerse in liquid, autoclave, or use harsh chemicals. The electrode pads are single-use only.
- Reprocessing: Not applicable. AEDs are not reprocessed between uses. After a single use/rehearsal, the pads and possibly the battery must be replaced per manufacturer instructions.
- Calibration: Internal calibration is performed automatically via self-tests. No user calibration is required.
- Storage:
- Store in an accessible, clearly marked location.
- Avoid extreme temperatures (<0°C or >50°C) and direct sunlight.
- Store in designated wall cabinets or carry cases.
- Ensure the status indicator shows a “ready” signal (usually a green light).
7. Procurement Guide
How to Select the Device
Consider your primary users and environment:
- For Public Access (PAD): Prioritize extreme simplicity, ruggedness, and clear voice prompts. A CPR feedback feature is highly valuable.
- For Professional/EMS Use: Consider models with manual override, ECG display, connectivity for data management, and compatibility with other equipment.
- Environment: For industrial or outdoor settings, choose a ruggedized model with a high IP rating.
Quality Factors
- Ease of Use: Simplicity of interface and clarity of prompts under stress.
- Reliability & Uptime: Robust self-testing regime and proven track record.
- Durability: Construction quality and warranty terms.
- Manufacturer Support: Availability of training, responsive technical support, and service network.
Certifications
Ensure the device holds the necessary regulatory approvals for your region: FDA 510(k) or PMA for the US, CE Mark under EU MDR for Europe, and other local approvals (e.g., CDSCO for India, TGA for Australia).
Compatibility
Ensure electrode pads and batteries are proprietary to the specific AED model and are readily available for purchase. For hospitals, check if the device can integrate with existing code cart systems or data management software.
Typical Pricing Range
- AED Unit: $1,200 – $2,500 USD.
- Long-Life Battery: $150 – $400.
- Adult Electrode Pads: $40 – $100 per set.
- Pediatric Pads/Key: $50 – $120.
- Wall Cabinet: $100 – $300.
- Total Program Cost: Factor in training, replacement parts, and potential connectivity fees.
8. Top 10 Manufacturers (Worldwide)
- Philips Healthcare (Netherlands/USA): A global leader with the HeartStart line, known for innovation and reliability.
- ZOLL Medical Corporation (USA, a subsidiary of Asahi Kasei): Renowned for its Real CPR Help® technology and professional AEDs.
- Stryker (USA) – Physio-Control: Maker of the iconic LIFEPAK series, with a strong presence in professional EMS markets.
- Defibtech (USA): Focuses on intuitive, high-quality AEDs like the Lifeline and Reviver series.
- Cardiac Science Corporation (USA, part of Stryker): Known for its Powerheart and G series AEDs with extensive self-testing.
- Nihon Kohden (Japan): A major player in Japan and Asia, offering a range of medical equipment including AEDs.
- Schiller AG (Switzerland): Produces the FRED easyport and other AEDs, strong in European markets.
- Metrax GmbH (Germany) – PRIMEDIC: Manufacturer of the PRIMEDIC Cube and other models, popular in Europe.
- Mindray (China): A rapidly growing global player offering cost-effective AEDs alongside a full medical equipment portfolio.
- BPL Medical Technologies (India): A leading Indian manufacturer, making AEDs more accessible in price-sensitive markets.
9. Top 10 Exporting Countries (Latest Year – Based on Recent Trade Data Trends)
Ranked by estimated export value of AEDs & parts.
- United States: Dominant exporter, home to major manufacturers (ZOLL, Physio-Control, Cardiac Science, Defibtech).
- Netherlands: Major European hub for Philips Healthcare’s global distribution.
- Germany: Home to strong exporters like Metrax (PRIMEDIC) and a hub for medical technology.
- China: Growing export power, driven by manufacturers like Mindray and as a global manufacturing center.
- Switzerland: Significant exporter via Schiller AG.
- Japan: Nihon Kohden and other manufacturers supply the advanced Japanese and Asian markets.
- Ireland: A key European distribution and manufacturing hub for several multinational med-tech companies.
- United Kingdom: Houses R&D and distribution centers for several global brands.
- Singapore: A major re-export hub for the ASEAN and Asia-Pacific regions.
- France: Home to production and distribution facilities for international players.
10. Market Trends
- Current Global Trends: Rapid proliferation in public spaces (PAD programs) and workplaces. Integration with Smart Cities & IoT, where AED locations are mapped in real-time for dispatch systems.
- New Technologies: Enhanced CPR feedback (real-time depth & rate). AI-powered rhythm analysis for even greater accuracy. Drones for AED delivery to remote or congested areas.
- Demand Drivers: Aging global population, increased incidence of cardiac conditions. Growing awareness and mandatory placement laws in schools, gyms, and public buildings. Corporate social responsibility (CSR) initiatives.
- Future Insights: The market will see more connected, data-driven AEDs that seamlessly transmit incident data to hospitals pre-arrival. Fully integrated emergency response ecosystems linking bystanders, 911, AEDs, and EMS. Continued focus on making devices even more intuitive and lowering cost barriers.
11. Training
Required Competency
While designed for use without training, formal Heartsaver or Basic Life Support (BLS) AED training is highly recommended. Competency includes:
- Recognizing cardiac arrest and activating emergency services.
- Performing high-quality CPR.
- Safely operating the specific AED model available.
- Coordinating CPR and defibrillation with other rescuers.
Common User Errors
- Delay in Use: Hesitating to start CPR or fetch the AED.
- Incorrect Pad Placement: Placing pads over clothing, jewelry, or medication patches.
- Not Following Prompts: Interrupting analysis by touching the patient or not clearing effectively before a shock.
- Stopping CPR: Pausing CPR for too long while retrieving or attaching the AED.
Best-Practice Tips
- Turn it ON: The first step—the device will guide you from there.
- Bare the Chest: Dry it, shave if necessary, remove patches.
- “Plug and Play”: Connect the pads if not pre-connected. Place one pad on the upper right chest, the other on the lower left side (apex).
- Clear and Shock: Ensure no one is touching the patient. Press the shock button only if advised.
- Resume CPR Immediately: After a shock, or if no shock is advised, immediately resume chest compressions. The AED will re-analyze every 2 minutes.
12. FAQs
1. Can I hurt someone or be sued for using an AED?
No. All 50 US states and many countries have Good Samaritan laws that protect lay rescuers who act in good faith during an emergency. It is far riskier to do nothing.
2. Will an AED shock someone who doesn’t need it?
No. The device only advises a shock for two specific, lethal rhythms (VF/VT). It cannot be overridden by an untrained user to deliver an unnecessary shock.
3. What’s the difference between adult and child pads?
Child pads deliver a reduced, pediatric-appropriate energy dose. Use them for children under 8 years or under 55 lbs (25 kg). If only adult pads are available, use them.
4. Can I use an AED on a person with a pacemaker?
Yes. Place the pads at least one inch away from the pacemaker implant (usually a small lump under the skin on the upper chest or abdomen).
5. Can I use an AED on a pregnant woman?
Yes. Saving the mother’s life is the priority. Use the standard AED procedure.
6. What if the victim is lying on a metal surface or in water?
Water conducts electricity. Move the victim away from standing water and dry the chest. A metal surface (like a bleacher) is generally safe, but ensure the electrode pads are only in contact with the victim’s skin, not the metal.
7. How often do I need to maintain the AED?
The device performs automatic self-checks. You should visually inspect the unit and its readiness indicator monthly. Replace pads and batteries as indicated by the device or by their expiration dates.
8. What should I do after using an AED?
Leave the pads attached and turn the AED over to arriving EMS personnel. They will download the data. Report what happened to the emergency responders.
13. Conclusion
The Automated External Defibrillator is a triumph of medical engineering—transforming a complex, hospital-based procedure into a simple, lifesaving action that anyone can perform. Its presence in public spaces and its integration into the Chain of Survival represents a fundamental shift in our ability to respond to sudden cardiac arrest, the leading cause of natural death in many parts of the world. By understanding what an AED is, how it works, and having the confidence to use it, you are not just reading a guide—you are potentially preparing to save a life. Remember the mantra: Call 911, Start CPR, Use an AED.
14. References
- American Heart Association. (2020). 2020 American Heart Association Guidelines for CPR and Emergency Cardiovascular Care.
- U.S. Food and Drug Administration (FDA). Device Classification Database.
- European Commission. Medical Device Regulation (MDR) 2017/745.
- International Organization for Standardization (ISO). ISO 60601-2-4: Medical electrical equipment – Part 2-4: Particular requirements for the basic safety and essential performance of cardiac defibrillators.
- Merchant, R. M., et al. (2020). “Part 1: Executive Summary: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care.” Circulation.
- World Health Organization (WHO). Medical Device Technical Series.
- Market research data synthesized from reports by Grand View Research, MarketsandMarkets, and Fortune Business Insights (2022-2024).