1. Definition
What is a Dialysis Water Treatment Monitor?
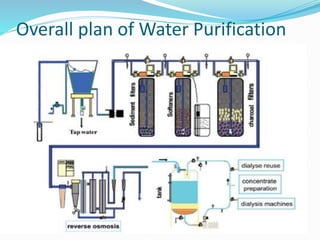
A Dialysis Water Treatment Monitor (often called a water quality monitor or water testing system) is a critical medical device used in hemodialysis units to continuously or periodically measure, analyze, and verify the chemical and microbiological purity of water used to prepare dialysate (dialysis fluid) and concentrates. Dialysis patients are exposed to 120-200 liters of water per week through the dialysate, making water quality paramount. Unlike a water treatment system (which purifies the water), the monitor is the quality assurance component. It acts as the vigilant “guardian,” ensuring the treated water meets the stringent standards set by international bodies like AAMI (Association for the Advancement of Medical Instrumentation), ISO, and pharmacopoeias. Failure to maintain these standards can lead to serious patient complications, including bloodstream infections, chemical toxicity, and chronic inflammation.
How it Works
The monitor works by sampling water from the treatment system’s distribution loop. It employs a combination of sensors and analytical methods:
- Continuous In-line Monitoring: Many parameters are measured in real-time using sensors placed directly in the water stream.
- Conductivity/Resistivity: The primary and most critical measurement. Pure water does not conduct electricity. Conductivity sensors measure the water’s ability to conduct an electric current, which increases with the concentration of ionized contaminants (like sodium, calcium, chloride). The inverse, resistivity (measured in MΩ·cm), is more commonly reported, with dialysis-grade water requiring ≥1 MΩ·cm.
- Total Chlorine: Uses amperometric sensors to detect even trace levels of chlorine and chloramines, which are highly toxic to dialysis patients.
- Temperature & Pressure: Monitored to ensure optimal conditions and system integrity.
- Periodic/Sample Testing: Some parameters require intermittent testing, often using separate bench-top analyzers or test kits.
- Bacterial & Endotoxin Testing: Performed by collecting water samples and culturing them for viable bacteria (using agar plates or rapid microbiological methods like ATP bioluminescence) or testing for endotoxins using the Limulus Amebocyte Lysate (LAL) assay.
- Chemical Contaminants: Specific ion meters (e.g., for fluoride, heavy metals) or photometric test kits are used periodically to verify compliance with chemical limits.
The monitor compares sensor readings against pre-set safety thresholds. If a parameter deviates from the acceptable range, the system triggers audible and visual alarms and may automatically divert water from the dialysis machines to drain (a “diversion valve” function) to protect patients.
Key Components
- Sensors/Probes: The core sensing elements (conductivity cell, chlorine sensor, temperature probe).
- Analyzer/Controller Unit: The “brain” that processes sensor signals, displays values (often on a digital screen), and houses the alarm logic.
- Alarm System: Visual (flashing lights) and audible alarms to alert staff.
- Sample Ports: Dedicated ports for aseptic collection of water for bacterial and endotoxin testing.
- Data Logging & Output: Internal memory or connectivity (USB, Ethernet) to record historical data for traceability and compliance reporting.
- Diversion Valve Interface: A relay or control output to command an external solenoid valve to divert unsafe water.
- Power Supply & Backup: Ensures continuous operation.
2. Uses
Clinical Applications
- Hemodialysis Centers: The primary application. Monitors ensure water used in central dialysis fluid delivery systems (CDDS) or individual machine proportioning meets AAMI/ISO quality standards (e.g., AAMI RD62:2022, ISO 23500-3).
- Home Hemodialysis: Compact, user-friendly monitors are essential for patient safety in home settings.
- Peritoneal Dialysis (PD): Water used to prepare PD solutions, especially in automated cyclers, must also meet strict purity standards.
- Dialysate & Concentrate Preparation: Monitoring water used in the on-site production of bicarbonate and acid concentrates.
Who Uses It
- Biomedical Technicians/Clinical Engineers: Responsible for installation, calibration, maintenance, and troubleshooting.
- Nephrology Nurses & Dialysis Technicians: Perform daily checks, respond to alarms, and collect samples for microbial testing.
- Infection Control Practitioners & Facility Managers: Review logs and reports to ensure systemic compliance.
Departments/Settings
- Hospital-based Nephrology & Dialysis Units
- Outpatient/Stand-alone Dialysis Clinics
- Home Healthcare Settings for home dialysis patients
3. Technical Specifications
Typical Specifications
- Resistivity/Conductivity Range: 0.1 – 18.2 MΩ·cm / 0.055 – 10 µS/cm.
- Accuracy: Typically ±1% of reading or ±0.1 µS/cm for conductivity.
- Total Chlorine Detection Limit: ≤0.01 mg/L (10 ppb), as per AAMI standards.
- Temperature Compensation: Automatic, referenced to 25°C.
- Alarm Set Points: User-configurable for high/low conductivity, high chlorine, etc.
- Outputs: 4-20 mA, relay contacts, digital (Modbus, Ethernet/IP).
- Power Supply: 100-240V AC, 50/60 Hz.
Variants & Sizes
- Wall-mounted/Panel-mounted Units: Common for central distribution monitoring.
- Portable/Bench-top Units: For spot-checking at various sampling points or in smaller clinics.
- Integrated Systems: Monitors built into the dialysis machine itself for final dialysate monitoring.
Materials & Features
- Materials: Sensors made of corrosion-resistant materials like 316L stainless steel, Pt1000 for temperature, PVC/PP flow chambers.
- Key Features: Touchscreen interfaces, multilingual support, extensive data logging with trend graphs, password protection, automatic temperature compensation, self-diagnostic functions.
Notable Models/Series
- Fresenius Medical Care: Aqua Monitor series.
- B. Braun: Diascan integrated systems, Water Monitoring Unit.
- Baxter: AK 98 monitor.
- Mar Cor (Cantel Medical): CMS-3T, PureFlow Monitor.
- Siemens: Sirocco water testing systems.
- GE Healthcare (now part of SUEZ): RO Check monitors.
4. Benefits & Risks
Advantages
- Enhanced Patient Safety: The foremost benefit, preventing exposure to contaminants that cause fever, sepsis, hemolysis, or long-term morbidity.
- Regulatory Compliance: Essential for meeting AAMI, ISO, and national regulatory standards.
- Process Control: Provides real-time feedback on the performance of the entire water treatment system (RO, deionizers, filters).
- Early Warning System: Detects issues like sanitization failure, filter breakthrough, or resin exhaustion before patients are affected.
- Documentation & Traceability: Data logging provides auditable proof of water quality.
Limitations
- Does Not Purify Water: It is a monitoring device only; it cannot correct water quality issues.
- Sensor Drift: Electrochemical sensors require regular calibration and maintenance for accuracy.
- Microbial Lag: Continuous sensors do not detect bacteria or endotoxins; these require separate, often time-consuming, tests.
- Initial & Ongoing Cost: Significant capital investment and recurring costs for consumables (sensors, reagents, culture media).
Safety Concerns & Warnings
- Alarm Fatigue: Improper set-up or frequent false alarms can lead to staff ignoring critical alerts. Never bypass or silence an alarm without investigating the root cause.
- Sensor Contamination: Fouling of probes by biofilm or scale leads to inaccurate readings. Adhere to a strict preventive maintenance schedule.
- Sample Contamination: Improper technique during microbiological sampling can yield false positives.
- Electrical Safety: Must be installed and grounded correctly, especially near water sources.
Contraindications
There are no direct contraindications for the monitor itself. However, dialysis should not be performed if the monitor indicates water quality is out of specification and the cause has not been identified and rectified. The device is contraindicated for use if it is out of calibration or has failed its performance verification.
5. Regulation
FDA Class
Class II (moderate to high risk). Subject to special controls (performance standards, post-market surveillance, guidelines). Requires 510(k) premarket notification.
EU MDR Class
Class IIb (devices used to administer or remove medicines, body liquids, or other substances to/from the body). Requires conformity assessment by a Notified Body.
CDSCO Category (India)
Class C (Moderate to High Risk). Regulated under the Medical Device Rules, 2017.
PMDA Notes (Japan)
Class II (Specified Controlled Medical Devices). Must comply with JP (Japanese Pharmacopoeia) standards for dialysis water and Pharmaceutical and Medical Device Act (PMD Act) requirements.
ISO/IEC Standards
- ISO 23500-3: Preparation and quality management of fluids for hemodialysis – Water for dialysis.
- ISO 13959: Water for hemodialysis and related therapies.
- ISO 26722: Water treatment equipment for hemodialysis applications.
- ISO 11663: Quality of dialysis fluid for hemodialysis.
- IEC 60601-1: General safety requirements for medical electrical equipment.
- IEC 60601-2-16: Particular requirements for hemodialysis equipment.
6. Maintenance
Cleaning & Sterilization
- External Housing: Wipe with a damp, soft cloth and mild detergent. Do not use abrasives or solvents.
- Sensor Chambers/Flow Cells: May require periodic disinfection with a chemical agent (e.g., peracetic acid, sodium hypochlorite) as per manufacturer’s instructions to control biofilm. Never autoclave the monitor or sensors.
Reprocessing
Not typically “reprocessed” like a reusable surgical device. The focus is on preventive maintenance of sensors and flow paths.
Calibration
- Conductivity: Must be calibrated regularly (weekly/monthly as per protocol) using certified traceable conductivity standard solutions (e.g., 100 µS/cm, 1000 µS/cm).
- Chlorine Sensor: Calibrated using standard chlorine solutions or ampoules.
- Temperature: Verified against a certified reference thermometer.
- Documentation: All calibration activities must be recorded in a log.
Storage
If taken out of service, the device should be stored in a clean, dry environment. Sensors, particularly chlorine probes, may require special storage solutions (e.g., a potassium iodide solution) to keep the electrode membrane hydrated.
7. Procurement Guide
How to Select the Device
- Assess Need: Determine if you need a central monitor, portable units, or integrated machine monitors.
- Compliance: Ensure the device is designed to meet AAMI/ISO standards for parameter range and accuracy.
- Alarm Functionality: Verify it has the required alarms and relay outputs to control a diversion valve.
- Ease of Use: Consider the user interface, training requirements, and clarity of alarms for clinical staff.
- Data Management: Evaluate data logging capacity, reporting features, and connectivity to hospital networks or dialysis management systems.
Quality Factors
- Sensor Longevity & Cost: Inquire about the expected lifespan and replacement cost of key sensors (e.g., chlorine sensors typically last 12-18 months).
- Manufacturer Support: Availability of local service engineers, technical support, and training.
- Ruggedness: Build quality suitable for a clinical environment.
- IP Rating: For wall-mounted units, an appropriate Ingress Protection (IP) rating for areas with potential splashes.
Certifications
Look for the CE Mark (for EU), FDA Listing (for USA), and certifications from other regional bodies (e.g., Health Canada, TGA Australia). ISO 13485 certification of the manufacturer is a strong indicator of a quality management system.
Compatibility
Must be compatible with your existing water treatment system’s plumbing (tube sizes, flow rates) and diversion valve. Verify communication protocol compatibility if integration with a Building Management System (BMS) is desired.
Typical Pricing Range
- Portable/Basic Monitor: $2,000 – $5,000 USD
- Advanced Central Monitoring System: $8,000 – $20,000+ USD
- Annual Maintenance & Consumables: $1,000 – $3,000 USD (sensors, calibration solutions, etc.)
8. Top 10 Manufacturers (Worldwide)
- Fresenius Medical Care (Germany/USA): Global leader in dialysis, provides integrated water systems and monitors.
- Baxter International (USA): Major player with dialysis machines and compatible water monitors.
- B. Braun (Germany): Offers the Diascan integrated monitoring and standalone water quality units.
- Nipro Corporation (Japan): Significant manufacturer of dialysis equipment, including water treatment components.
- Asahi Kasei (Japan): Parent company of Asahi Kasei Medical, produces dialysis machines and monitors.
- Cantel Medical (Mar Cor) (USA): Now part of STERIS, a leading specialist in water purification and monitoring for healthcare.
- SUEZ Water Technologies & Solutions (France/USA): (Formerly GE Water). Provides comprehensive water treatment and monitoring systems.
- Medtronic (Ireland): Through its Renal Care Solutions, offers monitoring technologies.
- Nikkiso (Japan): A key supplier of dialysis equipment globally.
- Siemens Healthineers (Germany): Provides water testing systems like Sirocco for analytical quality control.
9. Top 10 Exporting Countries (Latest Year)
(Based on HS Code 902780 for instruments for physical/chemical analysis)
- USA: Leading exporter of high-end medical monitoring and analytical devices.
- Germany: Renowned for precision engineering and medical technology (B. Braun, Siemens).
- Japan: Home to major players like Nipro, Asahi Kasei, and Nikkiso.
- China: Growing exporter of competitively priced monitoring devices and components.
- Ireland: Major medtech hub, with significant exports from companies like Medtronic.
- Switzerland: Home to many precision instrument manufacturers.
- Netherlands: Key European logistics and distribution hub for medical devices.
- France: Base for companies like SUEZ.
- Italy: Strong manufacturer of medical equipment components.
- United Kingdom: Exports specialized analytical and monitoring instruments.
10. Market Trends
Current Global Trends
- Rising Prevalence of ESRD: Increasing number of dialysis patients globally is the primary market driver.
- Stringent Regulations: Tighter AAMI/ISO standards are pushing for more sophisticated monitoring.
- Shift to Home Dialysis: Driving demand for compact, reliable, and user-friendly monitors for home use.
New Technologies
- Connectivity & IoT: Monitors with cloud connectivity for remote monitoring, predictive maintenance, and centralized data analytics across multiple clinics.
- Advanced Sensors: Development of more durable, low-maintenance chlorine sensors and probes resistant to biofilm fouling.
- Rapid Microbiological Methods (RMM): Integration of faster (hours vs. days) endotoxin and bacterial detection technologies into monitoring protocols.
Demand Drivers
- Aging populations and increasing diabetes/hypertension rates (leading causes of ESRD).
- Expansion of dialysis services in emerging markets (Asia-Pacific, Latin America).
- Growing awareness of water quality’s impact on patient outcomes.
Future Insights
The dialysis water monitor will evolve from a stand-alone alarm device to an intelligent node in a networked “smart dialysis ecosystem.” AI and machine learning will be used to predict sensor failure, optimize sanitization cycles, and provide actionable insights to improve overall water system management and patient safety.
11. Training
Required Competency
Operators must understand basic water treatment principles, the clinical importance of water purity, and the specific operation of the installed monitor. Competency includes performing checks, responding to alarms, collecting samples, and performing basic calibrations.
Common User Errors
- Ignoring or Silencing Alarms Without Investigation: A critical, potentially fatal error.
- Improper Calibration: Using expired or incorrect standard solutions, or not performing it regularly.
- Poor Sampling Technique: Contaminating microbiological test samples, leading to false positives and unnecessary system shutdowns.
- Neglecting Sensor Maintenance: Not cleaning or replacing sensors as scheduled, leading to inaccurate readings.
Best-Practice Tips
- Establish a Rigorous SOP: Create and follow detailed Standard Operating Procedures for daily checks, weekly/monthly maintenance, and alarm response.
- Maintain a Logbook: Meticulously document all readings, alarms, calibrations, maintenance actions, and microbial test results.
- Implement a “Two-Person Check” for critical steps like alarm response and post-sanitization testing.
- Foster a Culture of Safety: Empower all staff to “stop the line” if water quality is in doubt.
12. FAQs
- Q: How often should we test our dialysis water?
- A: Conductivity and chlorine must be monitored continuously. Chemical contaminant testing should be done at least annually. Bacterial cultures should be done at least monthly, and endotoxin testing at least quarterly, with more frequent testing recommended by many standards.
- Q: What is the most common cause of a conductivity alarm?
- A: A low resistivity (high conductivity) alarm is most often caused by exhausted reverse osmosis (RO) membranes or deionizer (DI) resin, or a malfunctioning RO system.
- Q: Our chlorine alarm keeps going off, but the city water report shows no chlorine. Why?
- A: The monitor detects total chlorine (free chlorine + chloramines). Many municipalities use chloramines, which are more stable. The alarm indicates a failure of the carbon tanks (which remove chloramines) to reduce levels below 0.1 mg/L.
- Q: Can we use the same monitor for our central system and our portable RO machines?
- A: Portable RO machines should have their own integrated monitor. A central monitor can be used to check water at the point of delivery, but each source needs independent validation.
- Q: How long can water sit in the distribution loop without being used?
- A: Stagnant water promotes biofilm growth. Loops should be designed for continuous recirculation. If a section is stagnant for >24 hours, it should be sanitized and flushed before use.
- Q: Who is responsible for maintaining the water monitor?
- A: While biomedical technicians handle technical maintenance, the entire dialysis team (nurses, techs, managers) shares responsibility for daily operational checks and responding appropriately to alarms.
- Q: Are “portable” water test strips sufficient for dialysis?
- A: No. Test strips are not sensitive or accurate enough to meet AAMI/ISO standards for critical parameters like chloramines or specific ions. They should never replace a calibrated, continuous monitoring system.
- Q: What should we do immediately if a water quality alarm sounds during a treatment?
- A: Follow your unit’s emergency protocol. Typically, this involves immediately stopping patient dialysis, diverting the water system, and transitioning patients to a safe alternative (e.g., saline flush per protocol). Then, investigate the cause.
13. Conclusion
The Dialysis Water Treatment Monitor is not merely a piece of equipment; it is a fundamental pillar of patient safety in hemodialysis. Its continuous vigilance against chemical and microbial contaminants is non-negotiable in preventing life-threatening complications. Success hinges on selecting the right technology, adhering to stringent international standards, implementing flawless maintenance and calibration routines, and fostering a culture where every alarm is treated as a potential threat. As dialysis technology advances, so too will the intelligence and connectivity of these monitors, further empowering clinics to deliver the safest possible care to a vulnerable patient population. Investing in a robust water quality monitoring program is, unequivocally, an investment in patient lives.
14. References
- Association for the Advancement of Medical Instrumentation (AAMI). ANSI/AAMI RD62:2022 Water for hemodialysis and related therapies.
- International Organization for Standardization (ISO). ISO 23500 series: Preparation and quality management of fluids for hemodialysis.
- Centers for Disease Control and Prevention (CDC). Guidelines for Preventing Healthcare-Associated Infections. Dialysis section.
- European Pharmacopoeia (Ph. Eur.). Monograph 01/2008:1167 “Water for Haemodialysis”.
- United States Pharmacopeia (USP). <1231> Water for Hemodialysis.
- Manufacturer technical manuals and operator guides from Fresenius, B. Braun, Mar Cor, etc.
- Note: Always consult the most current versions of standards and your local/national regulations, as they are subject to updates.