1. Definition
What is a Fetal Doppler?
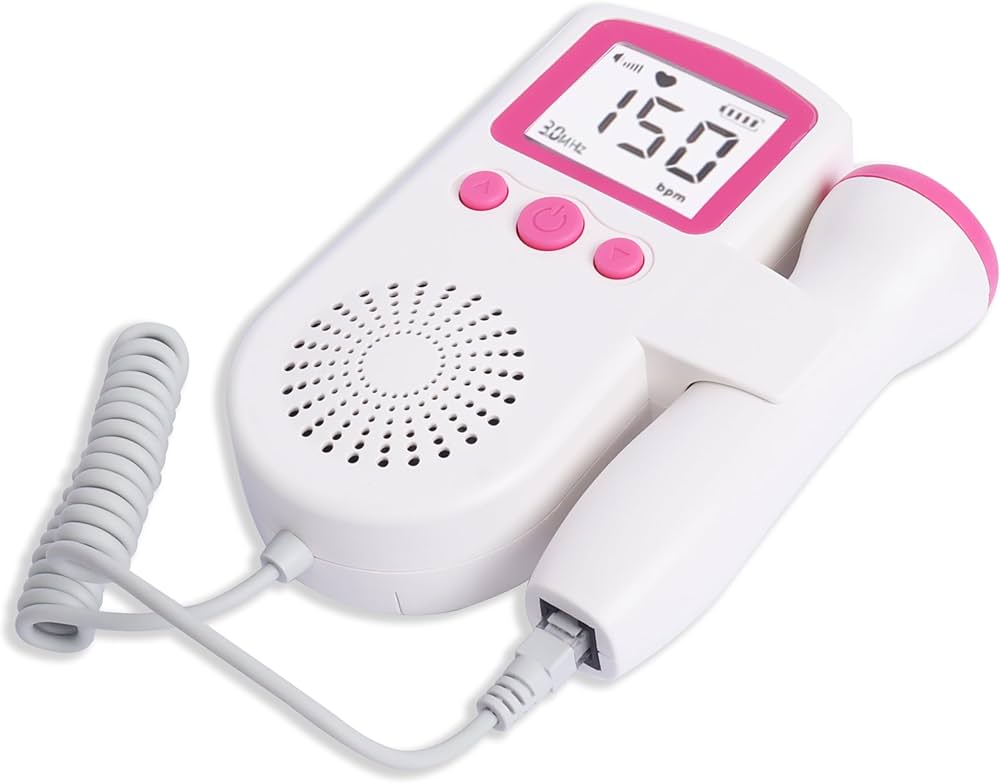
A fetal Doppler is a handheld, non-invasive medical device used to detect and monitor the fetal heartbeat during pregnancy. Using ultrasound technology, it amplifies the sound of the fetal heart, typically from around 10-12 weeks of gestation onward. Unlike imaging ultrasound, it focuses primarily on producing audible sound rather than visual images. It serves as a vital tool for both clinical reassurance and basic prenatal assessment, providing immediate auditory feedback about fetal well-being.
How it works
The device operates on the Doppler Effect principle. Here’s the simple breakdown:
- Transmission: A small probe (transducer) emits high-frequency, inaudible sound waves (typically 2-3 MHz) into the body.
- Reflection: These sound waves travel through tissue and fluid until they hit a moving object—in this case, the fetal heart.
- Frequency Shift: When the sound waves bounce off the moving heart valves and blood cells, their frequency changes. Movement toward the probe increases the frequency; movement away decreases it.
- Conversion & Amplification: The probe captures the reflected waves. The device’s internal electronics calculate the frequency shift, convert it into an audible sound, and amplify it through a speaker or headphones. The resulting “whooshing” sound represents the fetal heartbeat, and many devices also display the heart rate in beats per minute (BPM) on a digital screen.
Key Components
- Transducer/Probe: The handheld part placed on the abdomen. It contains piezoelectric crystals that generate and receive ultrasound waves. Probes are often waterproof for easy cleaning.
- Control Unit/Main Body: Houses the electronics, processor, and battery compartment. It interprets the signals and controls functions.
- Display Screen: An LCD or LED screen that shows the fetal heart rate (FHR) in BPM. Advanced models may show waveforms or have color displays.
- Audio Output: Includes a built-in speaker and often a headphone jack for private listening.
- Gel Port/Application Area: The surface of the probe where ultrasonic gel is applied to eliminate air between the probe and skin, ensuring efficient sound wave transmission.
- Battery: Usually rechargeable lithium-ion or standard AA/AAA batteries for portability.
- Cables & Connections: For linking the probe to the main unit (in non-integrated designs).
2. Uses
Clinical Applications
- Routine Prenatal Check-ups: Assessing fetal heart rate (FHR) as a standard vital sign during obstetric visits.
- Fetal Viability Confirmation: Verifying the presence of a fetal heartbeat, especially in the first and second trimesters.
- Fetal Well-being Monitoring: Evaluating fetal health in cases of decreased fetal movement, maternal concerns, or high-risk pregnancies (e.g., hypertension, diabetes).
- Labor Monitoring: In some settings, used intermittently alongside or prior to continuous electronic fetal monitoring (EFM) to assess the fetus during early labor.
- Multiple Gestations: Assisting in locating and differentiating between multiple fetal heartbeats.
Who uses it
- Obstetricians/Gynecologists (OB/GYNs)
- Midwives (both in hospitals and for home births)
- Prenatal Sonographers
- Labor & Delivery Nurses
- Family Practice Physicians
- Increasingly, expectant parents using OTC (Over-the-Counter) home Dopplers.
Departments/Settings
- Obstetrics & Gynecology Clinics
- Prenatal Care Units
- Labor & Delivery Wards
- Emergency Departments (for pregnant patients)
- Ultrasound/Diagnostic Imaging Departments
- Home Care Settings (with consumer-grade devices)
3. Technical Specs
Typical Specifications
- Ultrasound Frequency: 2 MHz (standard for later pregnancy), 3 MHz (common for early pregnancy), or dual-frequency probes (e.g., 2/3 MHz).
- Depth Penetration: Suitable for reaching the fetus through abdominal tissue.
- FHR Detection Range: Typically 50-240 BPM.
- Display: Digital FHR display; accuracy usually ±1-2 BPM.
- Audio Output: Built-in speaker, often with volume control and headphone output.
- Power Source: Rechargeable battery offering 2-8 hours of continuous use.
- Weight: 200-500 grams, designed for ergonomic handling.
Variants & Sizes
- Handheld/Portable Dopplers: Most common. Compact, battery-operated for easy use in clinics and homes.
- Desktop/Plug-in Dopplers: Used in clinical settings, often with more advanced features and connected to a power outlet.
- Hybrid Fetal Monitors: Combine Doppler technology with a tocograph to measure both FHR and uterine contractions, used primarily in labor wards.
Materials & Features
- Probe Casing: Made of medical-grade ABS plastic. The crystal face is often ceramic or composite.
- Gel: Use of water-based, hypoallergenic ultrasonic gel is mandatory.
- Key Features:
- Noise Reduction: Digital Signal Processing (DSP) to filter out background noise.
- Recording Capability: Ability to record heartbeat audio via app connectivity or USB.
- Multi-User Modes: Some clinical models support multiple probes.
- Waterproof Probe: For easy disinfection.
- Color Screens & Waveforms: Visual representation of the heartbeat pattern.
Models
Clinical models include EDAN F3 series, Newman Medical Fetal Doppler, and GE Healthcare’s Corometrics series. Popular OTC/home models include Sonoline B, Womb Music, and Baby Doppler brands.
4. Benefits & Risks
Advantages
- Non-invasive & Painless: No risk to mother or fetus when used appropriately.
- Immediate Reassurance: Provides quick confirmation of fetal cardiac activity, reducing anxiety.
- Portability & Ease of Use: Enables monitoring in various settings.
- Cost-Effective: Relatively inexpensive compared to full ultrasound systems.
- Strengthens Patient-Provider Bond: Allows parents to hear their baby’s heartbeat, enhancing the prenatal experience.
Limitations
- Not a Diagnostic Tool: Cannot assess fetal anatomy, position, or well-being comprehensively. A normal heartbeat does not rule out all potential issues.
- Operator Dependent: Finding the heartbeat requires skill and knowledge of fetal positioning.
- Early Pregnancy Limitation: Difficult to detect heartbeat before 10-12 weeks, leading to unnecessary worry.
- Can Mistake Sounds: Users may confuse the maternal aortic pulse or placental blood flow (whooshing) for the fetal heartbeat.
Safety Concerns & Warnings
- Thermal & Mechanical Index: While generally considered safe, the principle of ALARA (As Low As Reasonably Achievable) should guide use. Avoid prolonged, unnecessary use, especially in early pregnancy.
- Medical Reliance: Critical Warning: Home Dopplers should NOT be used to reassure or diagnose problems. If you have concerns about decreased movement or any other issue, seek immediate medical attention regardless of what you hear on the Doppler.
- Gel: Use only approved ultrasound gel. Other gels or lotions can damage the probe.
Contraindications
There are no absolute medical contraindications to its proper use. However, its use is misguided and dangerous if it delays seeking professional medical care for concerning symptoms.
5. Regulation
- FDA Class: Regulated as a Class II Medical Device in the United States. Requires 510(k) premarket notification to demonstrate substantial equivalence to a predicate device.
- EU MDR Class: Classified as a Class IIa Medical Device under the European Union Medical Device Regulation (MDR 2017/745).
- CDSCO Category: In India, falls under Class B (moderate to low risk) as per the Medical Device Rules, 2017.
- PMDA Notes: In Japan, regulated as a Class II Medical Device by the Pharmaceuticals and Medical Devices Agency (PMDA), requiring marketing approval.
- ISO/IEC Standards: Key standards include:
- IEC 60601-1: General safety for medical electrical equipment.
- IEC 60601-1-2: Electromagnetic compatibility.
- IEC 60601-2-37: Particular requirements for the basic safety and essential performance of ultrasonic medical diagnostic and monitoring equipment.
- ISO 13485: Quality management systems for medical device manufacturers.
6. Maintenance
Cleaning & Sterilization
- Between Patients (Clinical Use):
- Wipe probe with a soft cloth to remove gel.
- Disinfect: Use a low-level disinfectant wipe (e.g., 70% isopropyl alcohol or a quaternary ammonium compound) approved for medical equipment. Follow manufacturer’s instructions for contact time.
- Never immerse the main unit in liquid. The probe head can often be cleaned more thoroughly if waterproof.
- Home Use: Wipe the probe clean after each use.
Reprocessing
Fetal Dopplers are typically non-critical devices (contact with intact skin only). Therefore, high-level disinfection or sterilization is not required unless specified by the manufacturer or infection control policy (e.g., in a NICU setting).
Calibration
Clinical-grade devices require periodic performance checks and calibration (e.g., annually) as per the manufacturer’s guidelines and hospital policy to ensure accuracy of the FHR reading. This is usually done by a certified biomedical technician.
Storage
- Store in a clean, dry, temperature-controlled environment.
- Avoid extreme heat or cold.
- Store with the probe protected from impact.
- Ensure the battery is partially charged if storing for long periods.
7. Procurement Guide
How to Select the Device
Consider the primary user:
- For Clinical/Professional Use: Prioritize accuracy, durability, service contracts, and advanced features (e.g., high-quality speakers, connectivity to EMR).
- For Home/Personal Use (OTC): Prioritize ease of use, clear instructions, safety warnings, and good customer support. Look for FDA-cleared or CE-marked devices.
Quality Factors
- Detection Rate & Accuracy: Read reviews on ability to find heartbeat early and accuracy of FHR display.
- Battery Life: For portability.
- Build Quality: Should feel robust, not flimsy.
- Audio Clarity: Minimal static and background noise.
- Manufacturer Reputation & Support: Availability of technical support and warranty.
Certifications
- Mandatory: FDA 510(k) Clearance (US), CE Marking (EU), or equivalent national regulatory approval.
- Indicative of Quality: ISO 13485 certification of the manufacturing facility.
Compatibility
In clinical settings, check if the device can integrate with existing fetal monitoring systems or electronic medical records (EMR) via specific outputs or software.
Typical Pricing Range
- OTC/Home-Use Dopplers: $50 – $200 USD.
- Clinical/Professional-Grade Dopplers: $300 – $1,500+ USD, depending on features and brand.
8. Top 10 Manufacturers (Worldwide)
- GE Healthcare (USA)
- Profile: Global leader in medical imaging and monitoring.
- Product Line: Corometrics series of fetal monitors and Dopplers for professional use.
- Medline Industries, Inc. (USA)
- Profile: Major manufacturer and distributor of medical supplies.
- Product Line: Offers reliable clinical fetal Dopplers under various brands.
- EDAN Instruments, Inc. (China)
- Profile: Prominent global player in patient monitoring and diagnostics.
- Product Line: F3, F6, and other series of fetal Dopplers and monitors widely used worldwide.
- Bionet Co., Ltd. (South Korea)
- Profile: Specialized in fetal, maternal, and patient monitoring equipment.
- Product Line: Comprehensive range of handheld and modular fetal Dopplers.
- Huntleigh Healthcare (UK, part of Arjo)
- Profile: Long-standing specialist in fetal and vascular diagnostics.
- Product Line: Sonicaid series, a historic and trusted name in fetal Dopplers.
- BRAUN Medical (Germany, part of B. Braun)
- Profile: Diversified healthcare company with strong medical device divisions.
- Product Line: Offers fetal monitoring solutions including Dopplers.
- Contec Medical Systems (China)
- Profile: Growing manufacturer of monitoring and diagnostic devices.
- Product Line: Affordable and reliable fetal Dopplers for various markets.
- Bistos Co., Ltd. (South Korea)
- Profile: Manufacturer focused on patient monitoring and home care devices.
- Product Line: Range of portable fetal Dopplers.
- Newman Medical (USA)
- Profile: Supplier of simple, high-quality medical equipment.
- Product Line: Straightforward, durable handheld fetal Dopplers for professionals.
- Shenzhen Bestman Instrument Co., Ltd. (China)
- Profile: OEM/ODM manufacturer producing a vast number of devices for global brands.
- Product Line: Produces many of the white-label and budget fetal Dopplers sold online.
9. Top 10 Exporting Countries (Latest Year – Based on Recent Trade Data)
(Note: This is a generalized ranking based on medical device export patterns.)
- China: Dominates global exports as the manufacturing hub for both high-volume, lower-cost and mid-range devices.
- United States: Exports high-end, technologically advanced clinical Dopplers and monitors.
- Germany: Exports premium medical devices with a reputation for engineering excellence.
- South Korea: Major exporter of competitively priced, quality monitoring equipment.
- Netherlands: Acts as a key European distribution and re-export hub for medical technology.
- Japan: Exports specialized, high-quality devices, particularly in the professional segment.
- United Kingdom: Home to historical brands; exports specialized diagnostic equipment.
- Mexico: Significant exporter to the North American market, often via manufacturing partnerships.
- Malaysia: Growing ASEAN hub for medical device manufacturing and export.
- Italy: Exports niche medical devices and components for the broader monitoring sector.
10. Market Trends
Current Global Trends
- Rising Home-Use Market: Driven by increasing consumer awareness, prenatal bonding desires, and e-commerce accessibility.
- Telemedicine Integration: Development of Dopplers that can connect to smartphones and apps, allowing remote monitoring consultations.
- Point-of-Care Emphasis: Growth in portable devices for use in low-resource and community health settings.
New Technologies
- App Connectivity & Cloud Storage: Enables recording, sharing heartbeat audio, and tracking FHR over time.
- Advanced Signal Processing: AI and better algorithms to automatically locate heartbeat and filter noise more effectively.
- Wireless Probes: For greater patient comfort and clinician ease of use.
Demand Drivers
- Growing Global Birth Rates in certain regions.
- Increasing Prenatal Care Awareness.
- Rising Maternal Age and associated high-risk pregnancies requiring more monitoring.
- Consumerization of Healthcare: Patients taking a more active role in their health data.
Future Insights
The market will likely bifurcate further: highly sophisticated, connected clinical systems for professionals and simple, safe, app-enabled devices for consumers. Regulatory scrutiny on OTC devices may increase to ensure adequate safety warnings. The core value of the Doppler—providing an audible connection to the fetus—will remain its enduring appeal.
11. Training
Required Competency
- Anatomical Knowledge: Understanding of uterine size, fundal height, and typical fetal positions at various gestational ages.
- Probe Technique: Knowing how to apply gel, use minimal pressure, and employ a slow, systematic sweeping or rocking motion to locate the heartbeat.
- Auditory Discrimination: Ability to distinguish the fetal heartbeat (a fast, galloping sound, typically 120-160 BPM) from the maternal pulse (slower, synchronous with the mother’s own pulse) and placental souffle (a softer, whooshing sound).
Common User Errors
- Starting Too Early: Searching before 10-12 weeks and panicking when not finding the heartbeat.
- Using Insufficient Gel: This is the #1 reason for failure. Gel is essential for conduction.
- Pressing Too Hard: This can distort signals and cause discomfort.
- Misinterpreting Sounds: Mistaking the mother’s aortic pulse for the fetal heartbeat. Always correlate the heard rate with the mother’s own pulse.
- Giving Up Too Quickly: It can take several minutes of patient searching, especially with an active fetus or higher BMI.
Best-Practice Tips
- For best results after 12 weeks, have the mother lie semi-reclined with a slightly full bladder for early pregnancies (empty for later ones).
- Start low on the abdomen, just above the pubic bone, and angle the probe downward.
- Use a systematic approach: Move slowly upward and side-to-side.
- For home users: Set a time limit (e.g., 5 minutes). If you don’t find it, stop and try again later. Do not persist for hours. Never use it as a substitute for professional care.
12. FAQs
1. At what week can you first hear the heartbeat with a Doppler?
Most professionals can detect it reliably around 10-12 weeks. For home users, it’s common not to hear it until 12-14 weeks or later, especially for first-time mothers.
2. Is it safe to use a fetal Doppler every day?
While considered physically safe, daily use is not recommended medically or psychologically. It can lead to unnecessary anxiety if you have trouble finding the heartbeat. Use it sparingly for occasional bonding, not as a daily check-up.
3. Can the Doppler harm my baby?
Diagnostic ultrasound, including Doppler, has been used safely for decades. Following the ALARA principle, using it occasionally for short periods is not associated with harm. The greater risk is false reassurance.
4. I heard a heartbeat at 160 BPM, but my doctor found it at 140 last week. Is something wrong?
No. The fetal heart rate (FHR) normally varies between 110 and 160 BPM and can fluctuate with fetal activity, sleep cycles, and maternal activity. Variations are normal.
5. Why can’t I find the heartbeat?
Common reasons: too early in pregnancy, baby is in a difficult position, mother has a higher BMI, a full bladder is needed (early on), an empty bladder is needed (later on), or insufficient ultrasound gel.
6. My home Doppler shows a heart rate. Does that mean everything is fine with my baby?
No. A fetal Doppler only confirms cardiac activity at that moment. It does not assess fetal growth, anatomy, movement, oxygen levels, or the presence of other complications. It is a very limited tool.
7. Can I use aloe vera or lotion instead of ultrasound gel?
No. Only use approved, water-based ultrasound gel. Other substances can damage the probe’s crystal and do not provide proper acoustic conduction.
8. What should I do if I’m worried about my pregnancy but hear the heartbeat on my home Doppler?
Contact your healthcare provider immediately. Decreased movement, pain, bleeding, or any other concern trumps what you hear on a home device. Do not let the Doppler delay you.
13. Conclusion
The fetal Doppler is a remarkable tool that bridges the emotional and clinical aspects of prenatal care. In professional hands, it is a quick, efficient method for assessing a fundamental sign of fetal life. For parents, it offers a unique and profound moment of connection. However, this guide underscores a critical message: its simplicity is deceptive. It is not a comprehensive diagnostic device. Whether you are a clinician ensuring proper technique and maintenance, or an expectant parent considering a home device, understanding its capabilities, limitations, and risks is paramount. Used wisely, responsibly, and with clear knowledge, the fetal Doppler serves as a valuable adjunct to—never a replacement for—professional prenatal care.
14. References
- American College of Obstetricians and Gynecologists (ACOG). (2021). Ultrasound in Pregnancy.
- Food and Drug Administration (FDA). (2022). Consumer Updates: Avoid Fetal “Keepsake” Images, Heartbeat Monitors.
- International Electrotechnical Commission (IEC). IEC 60601-2-37: Particular requirements for the basic safety and essential performance of ultrasonic medical diagnostic and monitoring equipment.
- European Commission. Medical Device Regulation (MDR) 2017/745.
- World Health Organization (WHO). (2016). WHO recommendations on antenatal care for a positive pregnancy experience.
- Jago, J. R., et al. (2014). Equipment and safety in ultrasound and Doppler. In: Physical Principles of Doppler and B-mode Ultrasound.
- Market research reports from Grand View Research, Fortune Business Insights on the Fetal Monitor Market (2023-2030).
- Manufacturer user manuals and technical specifications from GE Healthcare, EDAN, and Newman Medical.