1. Definition
What is an Insulin Pump?
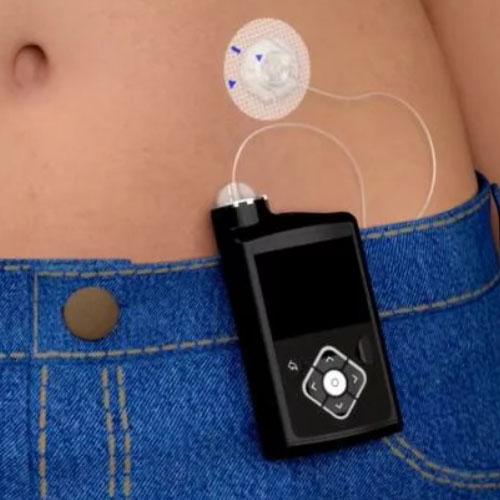
An insulin pump is a small, computerized medical device designed to deliver continuous subcutaneous insulin infusion (CSII) to individuals with diabetes mellitus. It serves as an alternative to multiple daily injections (MDI) with insulin pens or syringes. The pump’s primary function is to mimic, as closely as possible, the way a healthy pancreas releases insulin: providing a steady, low-level “basal” rate throughout the day and night, and allowing for on-demand “bolus” doses to cover meals and correct high blood glucose levels.
How it Works
The insulin pump is a programmable, battery-operated device. It holds a reservoir (or cartridge) of rapid-acting insulin. This reservoir is connected via a thin, flexible tube (infusion set) to a cannula—a tiny, flexible tube inserted just under the skin (usually in the abdomen, buttocks, or thigh). The pump is programmed by the user or a caregiver with personalized settings:
- Basal Rate(s): A tiny, pre-set amount of insulin delivered continuously (e.g., 0.5 to 2.0 units per hour). Multiple basal rates can be set for different times of day to match the body’s varying needs.
- Bolus Dose: A larger dose delivered at mealtime. The user calculates the dose based on carbohydrates consumed and current blood glucose, then commands the pump to deliver it.
- Correction Dose: An additional bolus to correct an unexpectedly high blood glucose reading.
The pump delivers insulin 24/7, providing more precise and flexible insulin management than injections.
Key Components
- Main Pump Unit: The external, pager-sized device containing the processor, user interface, and insulin reservoir chamber. It has a screen and buttons for programming.
- Insulin Reservoir/Cartridge: A small, disposable vial (typically holding 1.8 to 3.0 mL, or 180-300 units of insulin) that fits inside the pump.
- Infusion Set: A disposable assembly consisting of:
- Tubing: Connects the reservoir to the infusion site.
- Cannula: A thin, flexible (or sometimes steel) tube that remains under the skin. It is inserted using a needle, which is then removed.
- Adhesive Patch: Secures the infusion set to the skin.
- Battery: Usually a disposable AAA or rechargeable lithium-ion battery that powers the pump.
2. Uses
Clinical Applications
Insulin pumps are used for the management of:
- Type 1 Diabetes: The primary application, where the pancreas produces no insulin.
- Type 2 Diabetes: For individuals who have not achieved target glucose levels with MDI and have highly insulin-deficient profiles.
- Gestational Diabetes: In some cases where blood glucose is not controlled by diet and injectable insulin alone.
- Hyperglycemia in Hospitalized Patients: Increasingly used in ICU and non-ICU settings for safer and more effective glycemic control.
Who Uses It
- Patients: The primary users for daily self-management.
- Endocrinologists/Diabetologists: Prescribe and oversee pump therapy.
- Certified Diabetes Care and Education Specialists (CDCES): Provide critical training, education, and ongoing support.
- Nurses: Assist with inpatient pump management and patient education.
Departments/Settings
- Outpatient/Home Care: The most common setting.
- Endocrinology & Diabetes Clinics: For initiation, training, and follow-up.
- Hospital Wards & ICU: For continuous insulin infusion during hospitalization.
- Pediatric Diabetes Centers: Widely used for children and adolescents.
3. Technical Specs
Typical Specifications
- Dimensions: ~ 2″ x 3″ x 0.75″ (5 x 8 x 2 cm), similar to a small pager or smartphone.
- Weight: 80 – 120 grams (without insulin).
- Insulin Capacity: Reservoirs typically hold 1.8 mL (180 units) or 3.0 mL (300 units).
- Basal Increments: As fine as 0.01 to 0.05 units per hour.
- Bolus Increments: As fine as 0.05 to 0.1 units.
- Battery Life: 1-4 weeks, depending on model and usage.
Variants & Sizes
- Traditional/Tubed Pumps: The standard model with tubing connecting pump to infusion set.
- Patch Pumps (Tubeless): The reservoir and pump mechanism are housed in a small, disposable pod worn directly on the skin. A separate Personal Diabetes Manager (PDM) is used to wirelessly control it.
Materials & Features
- Materials: Durable plastics (polycarbonate, ABS), silicone, stainless steel cannulas.
- Key Features:
- Integrated Continuous Glucose Monitor (CGM): Sensor-augmented pumps that can display CGM readings.
- Suspend on Low (Low Glucose Suspend – LGS): Automatically stops insulin delivery if glucose falls to a preset threshold.
- Hybrid Closed-Loop (HCL) Systems: Advanced algorithms use CGM data to automatically adjust basal insulin delivery to keep glucose in range. The user still administers meal boluses.
- Water Resistance: Most are water-resistant for daily activities (e.g., IPX7 or IPX8 ratings).
Notable Models
- Medtronic: MiniMed™ 780G (HCL system), MiniMed™ 770G.
- Tandem Diabetes Care: t:slim X2™ with Control-IQ™ technology (HCL system).
- Insulet: Omnipod® 5 (tubeless, automated insulin delivery system).
- Ypsomed: mylife™ YpsoPump®.
- Roche: Accu-Chek® Solo (patch pump system).
4. Benefits & Risks
Advantages
- Improved Glycemic Control: Can lower HbA1c and reduce glucose variability.
- Flexibility: Easier management of variable schedules, meals, and exercise.
- Precision: Allows for very small, precise insulin doses, beneficial for children and highly insulin-sensitive individuals.
- Quality of Life: Reduces the burden of multiple daily injections.
- Advanced Features: Automated insulin delivery (HCL) can improve time-in-range and reduce hypoglycemia fear.
Limitations
- Cost: Significantly more expensive than injection therapy (device and ongoing supplies).
- Continuous Wear: Requires being attached to a device 24/7, which some find intrusive.
- Risk of DKA: If insulin delivery is interrupted (e.g., clogged cannula, dislodged set), ketosis can develop rapidly as no long-acting insulin is on board.
- Skin Issues: Possible irritation or infection at the infusion site.
Safety Concerns & Warnings
- Pump Malfunction: Alarms for occlusion (blockage), low battery, or empty reservoir must be heeded immediately.
- Site Infections: Strict site rotation and hygiene are crucial.
- User Error: Incorrect programming (e.g., wrong basal rate, bolus dose) is a major risk. Constant vigilance is required.
Contraindications
- Inability or unwillingness to perform frequent blood glucose monitoring (at least 4 times daily).
- Severe visual or hearing impairment that prevents operating the device.
- Inability to recognize or respond to hypoglycemia.
- Lack of commitment to follow the prescribed therapy and training.
5. Regulation
Insulin pumps are high-risk, active therapeutic devices and are tightly regulated globally.
- FDA Class: Class III (Premarket Approval – PMA required). Automated insulin delivery systems are regulated as Class II with special controls (de novo classification).
- EU MDR Class: Class IIb (rule 9 for devices administering medicinal products).
- CDSCO Category (India): Class C (moderate-high risk).
- PMDA (Japan): Regulated as Class III medical devices.
- ISO/IEC Standards:
- ISO 13485: Quality Management Systems for medical devices.
- ISO 60601-1: General safety for medical electrical equipment.
- ISO 60601-2-24: Particular requirements for the safety of infusion pumps and controllers.
- IEC 62304: Software lifecycle processes.
6. Maintenance
Cleaning & Sterilization
- The main pump unit is not sterilizable. Clean the exterior with a damp, soft cloth. Do not submerge or use harsh chemicals.
- Infusion sets, reservoirs, and cannulas are single-use, sterile, disposable items. They are not to be cleaned or reused.
Reprocessing
Not applicable. All components that contact insulin or pierce the skin are single-use.
Calibration
- Blood Glucose Meter Integration: If the pump has an integrated meter, the meter must be calibrated per its instructions.
- CGM Integration: CGM sensors require regular calibration with fingerstick blood glucose readings as per manufacturer guidelines.
- The pump’s internal delivery mechanism is factory-calibrated.
Storage
- Pump: Store at room temperature. Avoid extreme heat, cold, and moisture.
- Insulin: Store unopened (reserve) insulin in the refrigerator (2-8°C). Insulin in the pump reservoir is at room temperature and is typically used for no more than 2-3 days (check manufacturer guidelines).
- Supplies (Infusion Sets, Reservoirs): Store in a cool, dry place.
7. Procurement Guide
How to Select the Device
Consider: User’s age, dexterity, vision, lifestyle, diabetes management goals, and comfort with technology. Key decisions: Tubed vs. Tubeless, HCL capability, CGM compatibility, and waterproof rating.
Quality Factors
- Accuracy of Insulin Delivery: Verified by regulatory testing.
- Durability and Warranty: Typically 4 years.
- Ease of Use: Intuitive user interface and menu navigation.
- Customer & Technical Support: 24/7 helpline availability is critical.
- Data Management: Easy-to-use software for therapy review.
Certifications
Look for approvals from the relevant authority in your region: FDA (USA), CE Mark (EU), PMDA (Japan), etc.
Compatibility
- CGM Systems: Ensure the pump is compatible with your preferred CGM (e.g., Dexcom G6/G7, FreeStyle Libre 2/3).
- Data Software: Compatibility with clinic or personal data management platforms (e.g., Tidepool, Glooko).
Typical Pricing Range
- Pump Device: $4,000 – $8,000 (often covered partially/fully by insurance).
- Ongoing Monthly Supply Costs: $300 – $600 (for infusion sets, reservoirs, and CGM sensors).
8. Top 10 Manufacturers (Worldwide)
- Medtronic plc (Ireland/USA): The global market leader. Notable line: MiniMed™ series with Guardian™ CGM.
- Insulet Corporation (USA): Pioneer in tubeless pumps. Notable line: Omnipod®.
- Tandem Diabetes Care, Inc. (USA): Known for modern touchscreen pumps. Notable line: t:slim X2™ with Control-IQ™.
- Roche Diabetes Care (Switzerland): Offers integrated diabetes management solutions. Notable line: Accu-Chek® Solo.
- Ypsomed Holding AG (Switzerland): European leader. Notable line: mylife™ YpsoPump®.
- SOOIL Developments Co., Ltd. (South Korea): Major player in Asia. Notable line: DANA™ pumps.
- EoFlow (South Korea): Innovator in wearable, disposable pumps. Notable product: EOPATCH®.
- Dechra Pharmaceuticals PLC (Caressa) (UK/Netherlands): Dutch specialist in veterinary and human infusion. Notable line: Caressa®.
- F. Hoffmann-La Roche Ltd (Roche) – Old Product Lines: Still supports existing Accu-Chek® Spirit pumps.
- Cellnovo Group (UK/France): Developed a connected, touchscreen patch pump system (now part of PHC Holdings).
9. Top 10 Exporting Countries (Latest Year – 2023 Est.)
(Based on trends and industry reports)
- United States: The dominant exporter, home to Insulet, Tandem, and Medtronic’s operational HQ.
- Ireland: Major export hub for Medtronic’s manufacturing.
- Switzerland: Home to Roche and Ypsomed, key exporters to the EU and globally.
- South Korea: Growing export market led by SOOIL and EoFlow.
- Germany: Strong medical device manufacturing base and re-export hub.
- Netherlands: Distribution and manufacturing hub for European companies.
- United Kingdom: Home to Dechra and design centers for several companies.
- France: Has design and manufacturing facilities for key players.
- China: Emerging as a manufacturing and potentially export base.
- Japan: Produces and exports high-quality devices primarily for the Asian market.
10. Market Trends
- Current Global Trends: Rapid shift towards Automated Insulin Delivery (AID/Hybrid Closed-Loop) systems. Growth of tubeless/patch pumps. Increasing integration of data analytics and cloud connectivity for remote monitoring.
- New Technologies: Dual-hormone pumps (insulin + glucagon), fully closed-loop systems, implantable pumps, ultra-rapid insulin formulations for better pump performance.
- Demand Drivers: Rising global diabetes prevalence, technological advancements improving outcomes, growing patient awareness, and increasing reimbursement in developing markets.
- Future Insights: The market will be dominated by interoperable AID systems (where users can mix and match pump and CGM from different brands). Focus on miniaturization, greater automation, and AI-driven personalized insights. Potential for significant cost reduction making therapy more accessible.
11. Training
Required Competency
Users must be competent in: carbohydrate counting, blood glucose pattern recognition, bolus dose calculation, pump programming, infusion site management, and troubleshooting alarms and failures.
Common User Errors
- Missed Boluses: Forgetting to deliver insulin for a meal.
- Incorrect Carb Counting: Leading to over- or under-bolusing.
- “Site Run Too Long”: Not changing the infusion set every 2-3 days, leading to poor absorption or infection.
- Ignoring Occlusion Alarms: Leading to interrupted insulin delivery and risk of DKA.
- Programming Mistakes: Setting a temporary basal rate and forgetting to cancel it.
Best-Practice Tips
- Change your infusion set on time, every time. Rotate sites thoroughly.
- Always carry backup supplies: insulin, syringes/pens, infusion sets.
- Check blood glucose frequently, even with a CGM, to verify readings, especially for treatment decisions.
- Understand your pump’s alerts and alarms; never ignore them.
- Review pump and CGM data weekly with a caregiver or clinician to identify patterns.
12. FAQs
1. Can I shower or swim with an insulin pump?
Most traditional pumps are water-resistant and can be disconnected briefly for swimming, showering, or contact sports. Patch pumps (like Omnipod) are fully waterproof.
2. What happens if my pump breaks or fails?
All manufacturers provide a 24/7 helpline and will overnight a replacement pump. You must revert to insulin injections until the new pump arrives.
3. Will the pump know exactly how much insulin I need?
No. You program it with your settings. Even HCL systems require you to input meal carbs and confirm boluses. It’s not fully autonomous.
4. How often do I need to change the infusion set?
Typically every 2 to 3 days, or immediately if there is pain, redness, or the insulin isn’t working (high blood sugar).
5. Is pump therapy better than injections?
For many, it offers better control and flexibility, but it requires more commitment and education. It’s a personal choice best made with your healthcare team.
6. Can I travel with an insulin pump?
Yes. Carry it with you (do not check it in luggage). Inform airport security; pumps can usually go through body scanners (check your manufacturer’s guidelines).
7. How do I handle sick days?
You will have a “sick day” plan from your doctor, often involving more frequent glucose checks, ketone testing, and temporary basal rate adjustments.
8. Are insulin pumps covered by insurance?
In most developed countries, yes, if you meet certain criteria (e.g., type 1 diabetes, uncontrolled on injections). Coverage varies greatly by plan and region.
9. Can children use insulin pumps?
Absolutely. Pumps are widely used by children (even toddlers) and can offer parents better control and flexibility.
10. What’s the difference between a pump and an artificial pancreas?
A “hybrid closed-loop” pump is a step towards an artificial pancreas. It automates basal insulin. A true artificial pancreas (still in development) would also fully automate meal boluses.
13. Conclusion
The insulin pump represents a significant technological leap in diabetes management, transforming lives by offering precision, flexibility, and improved glycemic control. From traditional tubed pumps to advanced hybrid closed-loop systems and discreet patch pumps, the technology continues to evolve rapidly. Successful pump therapy hinges not just on the device itself, but on a triad of factors: a committed and trained user, a supportive healthcare team, and appropriate, ongoing education. While it requires a significant investment of time, money, and effort, for many individuals with diabetes, an insulin pump is a powerful tool that facilitates not just better health outcomes, but a better quality of life.
14. References
- American Diabetes Association. (2024). Standards of Medical Care in Diabetes. Diabetes Care.
- International Diabetes Federation. (2023). IDF Diabetes Atlas.
- U.S. Food and Drug Administration (FDA). Device Classification Databases.
- European Medicines Agency. European Union Medical Device Regulation (MDR) 2017/745.
- Medtronic, Tandem Diabetes Care, Insulet Corporation. (2024). Product Technical Manuals and User Guides.
- Khan, S., & Lieberman, J. A. (2023). The Evolution of Insulin Pump Technology. Journal of Diabetes Science and Technology.
- Grand View Research. (2024). Insulin Pumps Market Size, Share & Trends Analysis Report.
- ISO (International Organization for Standardization). ISO 13485:2016, IEC 60601-2-24:2012.