1. Definition
What is Point-of-Care Ultrasound (Handheld)?
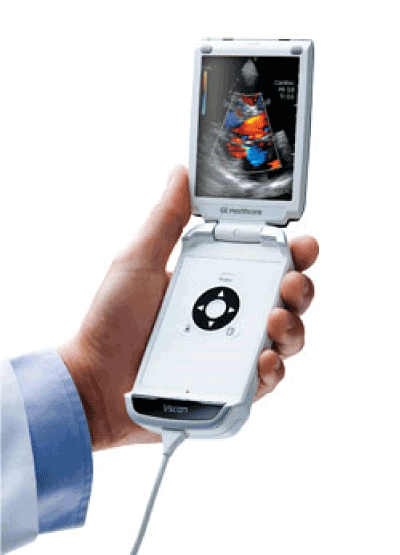
A Point-of-Care Ultrasound (POCUS) handheld device, often called a “pocket ultrasound” or “handheld ultrasound scanner,” is a compact, portable, and often wireless ultrasound machine designed for rapid diagnostic imaging at the patient’s bedside. It represents a revolutionary shift from traditional, cart-based ultrasound systems, bringing imaging capability directly into the hands of clinicians during patient encounters. Its primary function is to provide immediate, real-time visual information about a patient’s internal structures (like organs, blood vessels, and fluid) to guide clinical decision-making within seconds to minutes.
How it works
The fundamental principle is the same as all ultrasound: it uses high-frequency sound waves (inaudible to humans). The handheld device’s probe (transducer) emits these sound waves into the body. As the waves encounter different tissues and boundaries (e.g., between fluid and organ), they reflect back to the probe like an echo. The probe receives these returning echoes, and the device’s onboard computer processes the data. Using the known speed of sound in tissue and the time it takes for each echo to return, the software constructs a two-dimensional grayscale (or color, for Doppler) image in real-time on the device’s screen.
Key Components
- Transducer/Probe: The handheld unit itself, containing the piezoelectric crystal array that sends and receives sound waves. Most handhelds have a single, multi-frequency, general-purpose probe (e.g., convex or phased array) built-in.
- Display Screen: A built-in, high-resolution LCD or OLED touchscreen, typically 5 to 10 inches, for viewing images and controlling the device.
- Onboard Computer & Software: The processor and specialized software that handle image formation, processing, and measurement tools.
- Battery: A rechargeable lithium-ion battery that enables cordless operation for several hours.
- Connectivity Hardware: Wi-Fi, Bluetooth, and sometimes cellular modules for wirelessly transferring images to hospital networks or cloud storage.
- Controls: Physical buttons (e.g., power, freeze, depth/gain adjustment) and/or touchscreen interfaces for device operation.
2. Uses
Clinical Applications
- Emergency Medicine (ER): FAST exam (Focused Assessment with Sonography for Trauma) for internal bleeding, cardiac tamponade, pleural effusion, pneumothorax, AAA screening, and guiding procedures (e.g., vascular access, pericardiocentesis).
- Internal Medicine/Hospitalist: Assessment of cardiac function (e.g., LVEF, pericardial effusion), lung (B-lines for pulmonary edema, pneumothorax), deep vein thrombosis (DVT), and ascites.
- Critical Care (ICU/CCU): Hemodynamic monitoring (IVC collapsibility, cardiac output), ventilator management, procedural guidance (e.g., thoracentesis, paracentesis), and assessing line placement.
- Primary Care/Outpatient Clinic: Thyroid nodules, abdominal pain (gallstones, hydronephrosis), musculoskeletal issues (tendon tears, joint effusions), and basic obstetric dating.
- Anesthesiology: Nerve blocks, vascular access, and assessing gastric content.
- Sports Medicine & Rheumatology: Dynamic assessment of tendons, ligaments, muscles, and joints.
- Nephrology: Assessing kidney size, cysts, and hydronephrosis; guiding dialysis access.
- Obstetrics: Early pregnancy confirmation, fetal heart rate, and basic biometry (in skilled hands).
Who uses it
Physicians (across nearly all specialties), nurse practitioners, physician assistants, paramedics, and skilled midwives. The user base is defined less by title and more by specific, protocol-based training.
Departments/Settings
Emergency Departments, Intensive Care Units (ICUs), Operating Rooms, Labor & Delivery, Hospital Wards, Outpatient Clinics, Ambulances, Remote/Rural Health Posts, Sports Fields, and even in-home care.
3. Technical Specs
Typical Specifications
- Weight: 200g to 500g.
- Dimensions: Similar to a large smartphone or small tablet.
- Battery Life: 2 to 6+ hours of continuous scanning.
- Display: 5″ to 10″ touchscreen, sunlight-readable.
- Probe Frequency: Typically 2-5 MHz for abdominal/cardiac, with software tuning for other applications.
- Connectivity: Wi-Fi, Bluetooth, DICOM compatibility for PACS integration.
- Depth Penetration: Up to 30 cm, depending on tissue and probe.
- Water/Dust Resistance: Often IPX7 (can be submerged briefly) for easy cleaning.
Variants & Sizes
Variants are primarily defined by their probe type (convex/curvilinear for abdomen, phased array for cardiac, linear for vascular/MSK) and whether they are fully integrated (screen + probe in one) or a transducer that connects to a smartphone/tablet.
Materials & Features
- Materials: Medical-grade plastics, silicone grips, and acoustic lens material on the probe.
- Key Features:
- AI-Guided Imaging: Automated measurements (e.g., ejection fraction, bladder volume), image optimization, and anatomy identification.
- Cloud Integration: Secure image storage, sharing, and tele-consultation capabilities.
- Multiple Preset Modes: Abdominal, Cardiac, Lung, Vascular, OB/GYN, MSK with pre-optimized settings.
- Advanced Doppler: Color and Pulsed Wave Doppler on higher-end models for blood flow assessment.
Models (Notable Examples)
- Butterfly iQ+: A single-probe, chip-based system that connects to an iOS device.
- GE Healthcare Vscan Air: A pocket-sized, dual-probe (convex and linear) wireless device.
- Philips Lumify: A transducer that connects to an Android device/tablet with app-based software.
- Siemens Healthineers Acuson Freestyle/EchoBlaster: Handheld systems often used in cardiology and emergency care.
- Fujifilm Sonosite Iviz: A smartphone-sized handheld with a rotating probe head.
- Clarius HD3: Wireless, app-based scanners with a variety of probe types.
4. Benefits & Risks
Advantages
- Portability & Accessibility: Imaging is brought to the unstable patient, not vice versa.
- Rapid Diagnosis: Decisions are made in real-time, reducing time to diagnosis and treatment.
- Safety: No ionizing radiation, making it safe for repeated use and vulnerable populations.
- Cost-Effectiveness: Can reduce need for more expensive, time-consuming tests (e.g., CT scans).
- Procedural Guidance: Increases the success and safety of invasive procedures.
- Patient Engagement: Allows clinicians to show findings directly to patients, enhancing understanding.
Limitations
- Limited Field-of-View & Depth: Not suitable for deep abdominal imaging in obese patients or for comprehensive anatomic surveys.
- Operator Dependence: Image acquisition and interpretation are highly skill-dependent.
- Reduced Functionality: Lacks the power, advanced modes (like high-end Doppler, elastography), and transducer variety of high-end cart-based systems.
- Battery Life: Requires regular charging and management for continuous clinical use.
Safety Concerns & Warnings
- Thermal & Mechanical Indices: Users must be aware of on-screen TI/MI indices, especially in sensitive applications like early pregnancy or ocular scanning, and apply the ALARA principle (As Low As Reasonably Achievable).
- Infection Control: The device must be properly cleaned and disinfected between patients to prevent cross-contamination.
- Misdiagnosis Risk: Over-reliance on a limited POCUS exam without clinical correlation can lead to errors. It is a complementary tool, not always a definitive one.
Contraindications
There are no absolute device contraindications. Its use is contraindicated for a specific diagnostic purpose if:
- The operator lacks adequate training for that specific application.
- The clinical question requires a more comprehensive, formal ultrasound study.
- The anatomic region of interest is beyond the device’s penetration capability (e.g., detailed cardiac anatomy in a morbidly obese patient).
5. Regulation
Handheld POCUS devices are regulated as medical devices globally.
- FDA Class: Typically Class II (moderate to high risk), requiring a 510(k) premarket notification to demonstrate substantial equivalence to a predicate device.
- EU MDR Class: Generally Class IIa or IIb, depending on factors like duration of use and intended application (e.g., cardiac imaging often raises the classification).
- CDSCO Category: In India, they are categorized as Class B (moderate risk) or Class C (moderate-high risk) devices.
- PMDA Notes: In Japan, they are classified as Class II controlled medical devices. The PMDA emphasizes the importance of user training and clear labeling regarding intended use and limitations.
- ISO/IEC Standards:
- ISO 13485: Quality Management Systems for medical devices.
- IEC 60601-1: General safety requirements for medical electrical equipment.
- IEC 60601-2-37: Particular safety standards for ultrasound diagnostic equipment.
6. Maintenance
Cleaning & Sterilization
- Between Patients: Clean with a soft cloth dampened with mild soap and water or an EPA-approved low-level disinfectant wipe. Follow manufacturer instructions precisely.
- Important: Never submerge the main body unless specified (IPX7 rated). Avoid bleach, abrasive wipes, or solvents that can damage screens, plastics, or the acoustic lens.
Reprocessing
Handheld probes are generally non-sterile. For sterile procedures, a single-use, sterile probe cover must be used with appropriate acoustic gel inside the cover.
Calibration
Devices undergo factory calibration. User-level calibration is not typically required. Annual or bi-annual preventive maintenance by the manufacturer or certified technician is recommended to check acoustic output and system performance.
Storage
Store in a clean, dry, temperature-controlled environment (per manufacturer specs, often 0-40°C). Use a protective case. Ensure the battery is partially charged (40-80%) if storing for extended periods.
7. Procurement Guide
How to Select the Device
Consider: 1) Intended Use (ER vs. cardiology vs. MSK), 2) User Skill Level (AI assistance may help novices), 3) IT Infrastructure (Does it need to integrate with your PACS/EHR?), 4) Budget (include software subscriptions).
Quality Factors
- Image Clarity & Penetration: Test in realistic clinical scenarios (e.g., on a colleague with a larger body habitus).
- Ease of Use: Intuitive interface, quick boot-up, responsive touchscreen.
- Durability & Warranty: Look for drop ratings and length/terms of warranty.
- Software & Support: Frequency of updates, quality of customer/technical support, and training resources.
Certifications
Look for CE Mark (EU), FDA Clearance/Approval (US), and approvals from local regulatory bodies (e.g., CDSCO, PMDA, TGA). ISO 13485 certification of the manufacturer is a key quality indicator.
Compatibility
Ensure DICOM compatibility for image export and HL7 integration for EHR workflow. Check if it works with your institution’s preferred mobile OS (iOS/Android) and Wi-Fi security protocols.
Typical Pricing Range
Wide range: $2,000 – $15,000+ USD. Lower-cost models often use a subscription-based software model. Higher-end handhelds rival low-end cart prices but offer superior portability.
8. Top 10 Manufacturers (Worldwide)
- GE Healthcare (USA) – A titan in medical imaging. Their Vscan series is a leader in the handheld market, known for durability and image quality.
- Philips (Netherlands) – Lumify is their flagship handheld solution, renowned for its excellent image quality and robust app-based ecosystem.
- Butterfly Network, Inc. (USA) – Disruptor with the single-probe, semiconductor chip-based iQ+ system, popular for its affordability and extensive tele-education platform.
- Siemens Healthineers (Germany) – Offers the Acuson Freestyle and EchoBlaster lines, with strong capabilities in cardiac and emergency POCUS.
- Fujifilm Sonosite (USA/Japan) – A POCUS pioneer. Their iViz is a compact, rugged device born from their expertise in cart-based systems.
- Clarius Mobile Health (Canada) – Specializes in high-definition, app-based wireless scanners (HD3 series) with a focus on different probe types for specialties.
- Mindray (China) – A global patient monitoring and ultrasound giant. Their M9 and TE Air series offer high-performance handhelds at competitive prices.
- Samsung Medison (South Korea) – Leverages Samsung’s electronics expertise. The V7 and HERO series are feature-packed handhelds.
- Healcerion (South Korea) – Known for the SONON series, offering AI-powered, tablet-connected handheld systems.
- CHISON (China) – A major ultrasound manufacturer providing cost-effective handheld options like the iVis series.
9. Top 10 Exporting Countries (Latest Year – Based on Trade Data Trends)
(Ranked by estimated export value of ultrasound apparatus < 50kg, which encompasses handhelds)
- China – The world’s manufacturing hub, exporting massive volumes of devices across all price points.
- United States – A leader in high-tech, high-value exports from companies like GE, Butterfly, and Sonosite.
- Netherlands – Home to Philips, a major re-exporter and developer of medical technology.
- South Korea – Strong exports from Samsung Medison and Healcerion, known for technological innovation.
- Germany – High-quality engineering exports from Siemens Healthineers and other med-tech firms.
- Japan – Home to Fujifilm and other precision manufacturers, exporting advanced devices.
- Canada – Notable for Clarius’s specialized wireless scanner exports.
- Italy – A significant European manufacturer and exporter of medical devices.
- United Kingdom – Hosts R&D and headquarters for several innovative medical tech companies.
- France – Has a established medical device sector contributing to global exports.
10. Market Trends
- Current Global Trends: Explosive growth driven by decentralization of healthcare, rising prevalence of chronic diseases, and emphasis on value-based care. Post-COVID, demand for portable, easy-to-clean devices surged.
- New Technologies: Artificial Intelligence (AI) is the biggest driver, automating measurements, guiding novice users, and enhancing image interpretation. Cloud computing enables seamless data management and tele-ultrasound.
- Demand Drivers: Growing adoption by non-radiologists, increasing procedural volumes requiring guidance, expansion into low-resource settings, and patient demand for faster diagnostics.
- Future Insights: The line between handheld and cart-based systems will blur as handhelds gain more power and features. Multi-modal integration (e.g., ultrasound with digital stethoscope) may emerge. AI will evolve from an assistant to a potential autonomous screening tool in specific applications.
11. Training
Required Competency
Competency is application-specific. A clinician should be formally trained in the image acquisition, interpretation, and clinical integration for each specific use (e.g., FAST exam, lung ultrasound, basic echocardiography). This typically involves didactic courses, supervised hands-on scanning, and quality assurance review.
Common User Errors
- Probe Pressure: Pressing too hard (displaces structures) or too lightly (poor contact).
- Ignoring Machine Settings: Not adjusting depth, gain, or frequency for the specific patient or structure.
- Over-interpretation: Diagnosing artifacts (e.g., reverberation artifacts as pathology).
- Scope Creep: Using the device for an application beyond one’s training.
- Poor Ergonomics: Leading to sonographer injury and unstable imaging.
Best-Practice Tips
- Start with a System: Always follow a standardized scanning protocol for the clinical question.
- Optimize Before You Diagnose: Spend 10 seconds adjusting depth and gain—it transforms image quality.
- Correlate, Correlate, Correlate: Integrate ultrasound findings with the patient’s full clinical picture.
- Know Your Limits: Document “limited study” when conditions are suboptimal and know when to refer for a formal study.
- Practice Regularly: Skill decays without use. Scan colleagues and normal patients to build a mental library of “normal.”
12. FAQs
1. Is a handheld ultrasound as good as a big machine?
For its intended purpose—rapid, focused, bedside assessment—it is excellent. For a comprehensive, detailed anatomical survey (e.g., a full fetal anomaly scan), a high-end cart system with multiple probes is superior.
2. Can anyone buy and use one?
While commercially available, effective and safe use requires specific medical training. Misuse can lead to misdiagnosis and patient harm.
3. How long does it take to learn POCUS?
Basic applications (e.g., FAST, lung B-lines) can be learned in a focused 1-2 day course with ongoing practice. Complex applications (e.g., cardiac function) require extensive, longitudinal training.
4. Are the images legally part of the medical record?
Yes. They must be saved, archived, and retrievable like any other diagnostic study, usually via DICOM export to your hospital’s PACS.
5. What’s the difference between a “handheld” and a “portable” ultrasound?
“Portable” often refers to laptop-sized systems on wheels with more features and separate probes. “Handheld” or “pocket” refers to the all-in-one, phone/tablet-sized devices.
6. How do I manage patient data privacy with these devices?
Only use devices with secure, encrypted data transmission. Never store patient images on personal smartphones or unsecured clouds. Use institutional IT-approved workflows.
7. What about software updates and subscriptions?
Many models operate on a software subscription (SaaS) model. Factor this ongoing cost into procurement and ensure updates are included for safety and new features.
8. Can I use it on a patient with a pacemaker or implant?
Yes, ultrasound is safe with implants. The implant may cause acoustic shadowing, obscuring structures behind it.
9. How many exams can I do on one charge?
Varies by model and scan duration. Typically 10-30+ focused exams. Always have a charging routine (e.g., charge at lunch, after shift).
10. Is Doppler function important on a handheld?
It’s very useful for confirming vascular access, assessing DVT, and basic cardiac flow assessment. It’s a key differentiator between basic and advanced handheld models.
13. Conclusion
The handheld point-of-care ultrasound device is more than just a miniaturized scanner; it is a fundamental extension of the modern clinician’s physical exam. It democratizes diagnostic imaging, allowing for immediate, non-invasive insight into the body’s inner workings. While not a replacement for comprehensive imaging or clinical judgment, it is a powerful, safe, and transformative adjunct tool. Its successful implementation hinges on selecting the right device for the clinical need, investing in structured, ongoing user training, and integrating it responsibly into clinical workflow. As technology advances, particularly with AI, the role of handheld POCUS will only expand, solidifying its place as an essential tool in 21st-century medicine.
14. References
- American Institute of Ultrasound in Medicine (AIUM). (2023). Practice Guidelines for Performance of Point-of-Care Ultrasound.
- International Federation for Emergency Medicine (IFEM). (2020). Point-of-Care Ultrasound Curriculum Guidelines.
- U.S. Food and Drug Administration (FDA). (2023). Marketing Clearance of Diagnostic Ultrasound Systems.
- Moore, C. L., & Copel, J. A. (2011). Point-of-Care Ultrasonography. New England Journal of Medicine.
- Global Market Insights. (2023). Handheld Ultrasound Device Market Report.
- Manufacturer Technical Specifications and User Manuals (GE, Philips, Butterfly, Siemens, etc.).
- International Electrotechnical Commission (IEC). IEC 60601-2-37: Medical electrical equipment – Part 2-37: Particular requirements for the basic safety and essential performance of ultrasonic medical diagnostic and monitoring equipment.