1. Definition
What is a TENS Unit?
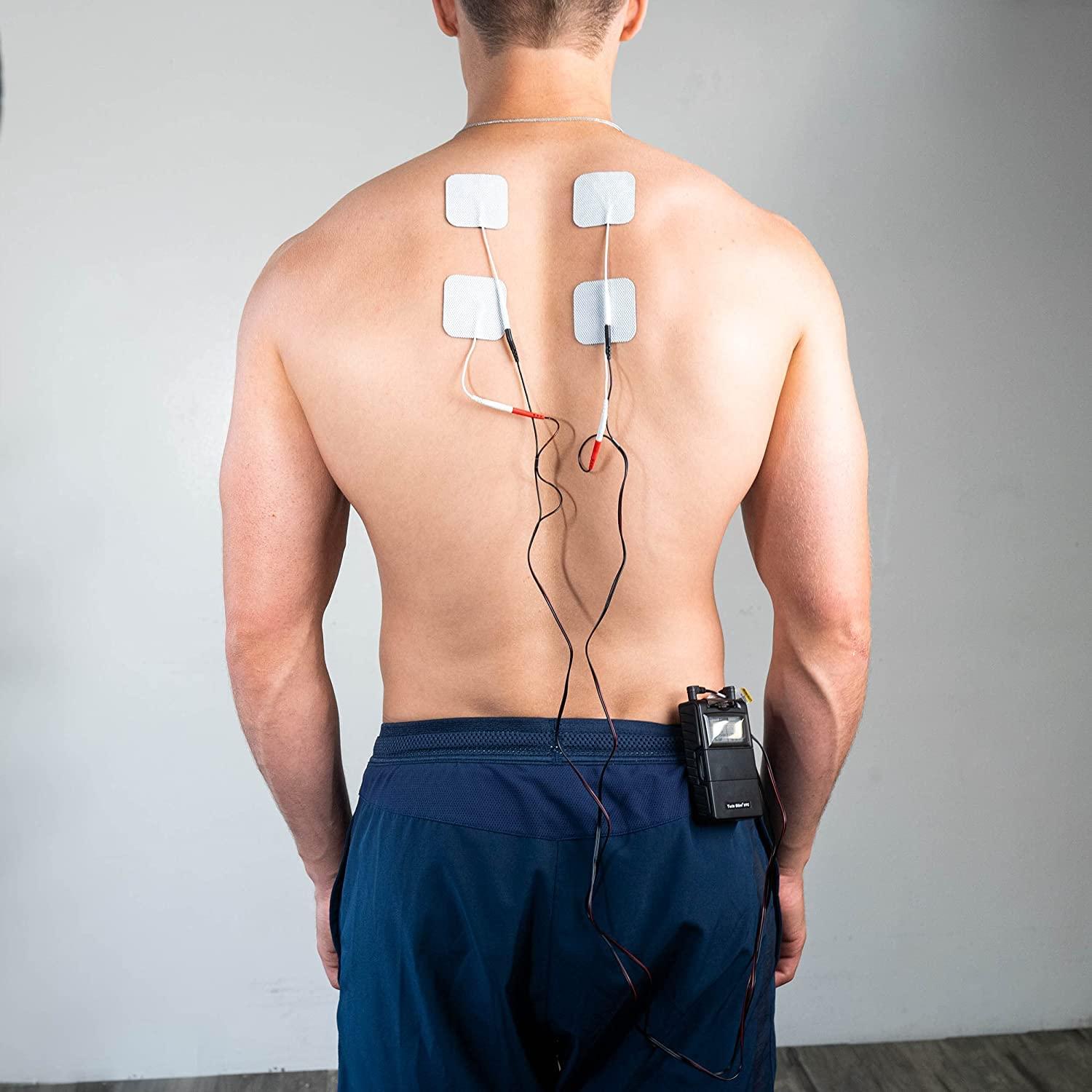
A Transcutaneous Electrical Nerve Stimulation (TENS) unit is a portable, battery-powered electronic device used primarily for pain management in physiotherapy and at-home care. It delivers mild, low-voltage electrical currents through electrodes placed on the skin’s surface. The primary function of a TENS unit is to provide non-invasive, drug-free pain relief by modulating pain signals to the brain and stimulating the body’s natural painkillers, known as endorphins.
How it Works
TENS works on two primary neurophysiological principles:
- Gate Control Theory of Pain: The electrical impulses generated by the TENS unit travel along the nerve fibers faster than pain signals. These impulses effectively “close the gate” at the spinal cord level, preventing pain signals from reaching the brain. This is often used for acute, localized pain with a high-frequency, low-intensity setting.
- Endorphin Release: Lower-frequency electrical stimulation encourages the brain to release endorphins and enkephalins, the body’s natural opioids. This provides a more generalized, longer-lasting analgesic effect, suitable for chronic pain conditions.
Additionally, some modern units incorporate Microcurrent Electrical Nerve Stimulation (MENS) or Neuromuscular Electrical Stimulation (NMES) modes, which can aid in reducing inflammation and stimulating muscle contractions, respectively.
Key Components
- Main Unit/Generator: The compact, handheld device containing the battery and microprocessor that generates the electrical pulse. It features control buttons and a digital display.
- Electrodes: Adhesive pads that are placed on the skin. They conduct the electrical impulses from the unit to the body. Electrodes come in various sizes and shapes for different treatment areas.
- Lead Wires: Insulated cables that connect the electrodes to the main unit. Some modern devices use wireless Bluetooth connectivity between the unit and electrodes.
- Battery: Typically rechargeable lithium-ion or replaceable AAA/AA batteries that power the device.
- Controls & Interface: Buttons or a touchscreen to adjust key parameters: Pulse Rate/Frequency (Hz: how many pulses per second), Pulse Width (µs: duration of each pulse), and Amplitude/Intensity (mA: strength of the current).
2. Uses
Clinical Applications
TENS is used to manage both acute and chronic pain conditions, including:
- Musculoskeletal Pain: Osteoarthritis, rheumatoid arthritis, lower back pain, neck pain, tendinitis, bursitis.
- Neuropathic Pain: Diabetic neuropathy, post-herpetic neuralgia (shingles), phantom limb pain, radiculopathy (e.g., sciatica).
- Post-Surgical Pain: As an adjunct to medication following orthopedic, abdominal, or thoracic surgeries.
- Labor Pain: Used as a non-pharmacological method for pain relief during childbirth.
- Sports Injuries: Pain management for sprains, strains, and contusions.
- Rehabilitation: To reduce pain during therapeutic exercises and improve range of motion.
Who Uses It
- Physiotherapists / Physical Therapists: The primary professionals prescribing and administering TENS in clinical rehabilitation.
- Chiropractors and Osteopaths: For pain management in manual therapy practices.
- Pain Management Specialists and Anesthesiologists: In multidisciplinary pain clinics.
- Occupational Therapists: To manage pain that interferes with daily activities.
- Sports Medicine Physicians and Athletic Trainers.
- Patients: For continued, prescribed home use under professional guidance.
Departments/Settings
- Outpatient Physiotherapy & Rehabilitation Clinics
- Hospital Pain Management and Physiotherapy Departments
- Orthopedic and Sports Medicine Centers
- Chiropractic Clinics
- Women’s Health Units (for labor pain)
- Home Healthcare Settings
3. Technical Specs
Typical Specifications
- Pulse Frequency (Rate): Adjustable, typically 1-150 Hz. (Conventional: 80-150 Hz; Acupuncture-like: 1-10 Hz).
- Pulse Width (Duration): 50 to 250 microseconds (µs).
- Output Current: Adjustable amplitude, usually 0-80 mA (per channel).
- Modulation Modes: Burst, modulation, and constant modes to prevent nerve accommodation.
- Channels: Most units are dual-channel, allowing two pairs of electrodes to be used simultaneously.
- Battery Life: 20-40 hours of typical use on a single charge.
Variants & Sizes
- Clinical/Professional Models: Larger, more robust units with advanced programming, more modulation modes, and higher output.
- Portable/Consumer Models: Compact, lightweight, user-friendly devices designed for home use. Some are as small as a smartwatch or a card deck.
- Wireless TENS: The electrode pads contain mini-receivers and are connected wirelessly (via Bluetooth) to a small controller or smartphone app.
Materials & Features
- Construction: Durable medical-grade plastic casing. Electrodes are made of conductive carbonized silicone or hydrogel with a medical-grade adhesive.
- Special Features: Pre-programmed pain relief modes (e.g., “Back,” “Arthritis,” “Muscle”), LCD screens, timer functions, intensity ramping, and water resistance (IPX ratings). Advanced models may offer Interferential Current (IFC) therapy, which uses two medium-frequency currents to penetrate deeper tissue.
Notable Models
- Omron Max Power Relief: Popular consumer dual-channel device.
- HealthmateForever YK15AB: Known for multiple modes and affordability.
- Compex: Offers premium devices with NMES and TENS combinations for sports recovery.
- Alevia (by Zimmer MedizinSystems): Professional-grade device often used in clinics.
- TENS 7000: A classic, no-frills model favored for its simplicity and effectiveness.
4. Benefits & Risks
Advantages
- Non-Invasive and Drug-Free: Reduces reliance on pain medication and associated side effects.
- Portable and Easy to Use: Enables continuous pain management at home, work, or while traveling.
- Safe with Minimal Side Effects: When used correctly, adverse effects are typically limited to minor skin irritation.
- Cost-Effective: One-time purchase (aside from electrode replacements) compared to ongoing medication costs.
- Patient-Controlled: Empowers patients to actively manage their pain by adjusting intensity as needed.
Limitations
- Variable Efficacy: Pain relief is subjective and may not work for everyone or for all types of pain.
- Temporary Relief: Often provides symptomatic relief during and shortly after use, rather than a cure.
- Placement-Dependent: Incorrect electrode placement can render treatment ineffective.
Safety Concerns & Warnings
- Electrode Placement: Never place electrodes over the eyes, front/side of neck, mouth, or head. Avoid placement over broken, irritated, or numb skin.
- Intensity: Increase slowly to a strong but comfortable sensation. A painful or burning feeling indicates the intensity is too high.
- Use During Activities: Do not use while driving, operating machinery, or in the bath/shower unless the device is specifically rated for water immersion.
- Device Malfunction: Discontinue use if the unit feels hot, emits a strange odor, or causes unexpected pain.
Contraindications
- Pacemaker or Implantable Defibrillator Users: Electrical interference can disrupt device function.
- Pregnancy (First Trimester & Certain Areas): Avoid abdominal and pelvic placement unless specifically for labor pain under medical supervision.
- Epilepsy: Stimulation over the head or neck may trigger a seizure.
- Active Cancer or Tumors: Do not place electrodes over or near malignant tissue.
- Deep Vein Thrombosis (DVT): Stimulation could theoretically dislodge a clot.
- Cardiac Disease: Use with extreme caution and only under physician guidance.
5. Regulation
FDA Class
In the United States, TENS units are regulated by the FDA as Class II medical devices. They require a 510(k) premarket notification to demonstrate substantial equivalence to a legally marketed predicate device.
EU MDR Class
Under the European Union Medical Device Regulation (MDR), TENS units are classified as Class IIa medical devices. They require a CE marking issued by a Notified Body following a conformity assessment.
CDSCO Category
In India, the Central Drugs Standard Control Organization (CDSCO) classifies TENS units under Class B medical devices (moderate-low risk) as per the Medical Devices Rules, 2017.
PMDA Notes
Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) regulates TENS as Class II medical devices. They require certification from a Registered Certification Body (RCB).
ISO/IEC Standards
Key applicable standards include:
- ISO 13485: Quality management systems for medical device manufacturing.
- IEC 60601-1: General safety requirements for medical electrical equipment.
- IEC 60601-2-10: Particular safety requirements for nerve and muscle stimulators.
6. Maintenance
Cleaning & Sterilization
- Main Unit: Wipe with a soft, damp cloth. Do not immerse in liquids or use harsh chemicals.
- Electrodes: Most are single-patient use and disposable. Reusable electrodes can be cleaned with a mild soap and water solution after each use to remove skin oils and gel residue. Allow to air dry completely before reattaching the lead wires.
Reprocessing
Lead wires are considered single-patient use items in clinical settings or should be periodically inspected for wire breakage or insulation damage in home settings.
Calibration
Consumer TENS units do not require user calibration. Professional-grade devices may have calibration checks performed annually by the manufacturer or an authorized service provider to ensure output accuracy.
Storage
Store the device and electrodes in a cool, dry place away from direct sunlight and extreme temperatures. Remove batteries if the device will not be used for an extended period. Keep electrodes in their sealed package until use to prevent the adhesive from drying out.
7. Procurement Guide
How to Select the Device
Consider the end-user (clinic vs. patient), intended conditions, required portability, and desired features (e.g., pre-set programs, wireless capability).
Quality Factors
- Output Stability: Should deliver consistent pulse form and intensity.
- Durability: Robust construction and a good warranty period.
- Ease of Use: Intuitive controls and a clear display.
- Electrode Quality: Look for hypoallergenic, long-lasting adhesive electrodes.
Certifications
Always verify FDA clearance/approval (USA), CE Marking (EU), or other relevant regional regulatory approvals. ISO 13485 certification of the manufacturer is a strong quality indicator.
Compatibility
Ensure lead wire connectors match the electrodes (standard 2mm or 3.5mm pin connectors are common). For clinic integration, consider if data logging or compatibility with other electrotherapy devices is needed.
Typical Pricing Range
- Basic Consumer Models: $30 – $80 USD
- Advanced Consumer/Wireless Models: $80 – $200 USD
- Professional/Clinical Models: $200 – $600+ USD
- Electrodes (pack of 4): $10 – $25 USD (reusable types last 15-30 uses).
8. Top 10 Manufacturers (Worldwide)
- Omron Corporation (Japan): A global electronics giant with a strong line of consumer health devices, including reliable TENS units.
- DJO Global (Enovis) (USA): A leading provider of orthopedic solutions, offering professional-grade TENS devices under brands like Chattanooga and Empi.
- Zimmer MedizinSystems (Germany): Known for its Alevia series, widely used in European physiotherapy clinics.
- Compex (A DJO Global Company) (Switzerland/USA): Specializes in high-end electrical muscle and nerve stimulators for athletic and clinical markets.
- RS Medical (USA): Focuses on prescribed home-use stimulators for chronic pain, often involving longer rental periods.
- NeuroMetrix, Inc. (USA): Developer of Quell, a wearable, prescription-strength neurostimulation device worn on the calf.
- Pure Enrichment (USA): A popular brand for affordable, user-friendly consumer wellness devices, including TENS.
- HealthmateForever (China): A major manufacturer producing a wide range of TENS units sold under various brand names globally.
- Beurer GmbH (Germany): A well-known European manufacturer of health and wellness products, offering several TENS models.
- I-Tech Medical Division (USA): Manufactures the industry-standard Dynatronic TENS and IFC devices for clinical use.
9. Top 10 Exporting Countries (Latest Year – 2023 Data Estimates)
Ranked by estimated export value of electrotherapy apparatus including TENS.
- China: The dominant global manufacturer and exporter, offering a vast range from low-cost to mid-tier devices.
- United States: A major exporter of high-end, professional, and innovative neurostimulation devices.
- Germany: Exports high-quality, precision-engineered medical devices, including premium TENS units.
- Mexico: A significant exporter, often housing manufacturing plants for major US corporations (e.g., DJO).
- Switzerland: Home to specialized manufacturers like Compex, exporting premium sports recovery devices.
- Japan: Exports advanced electronics integrated into TENS devices from companies like Omron.
- Netherlands: A key EU distribution and export hub for medical devices.
- Ireland: Hosts numerous med-tech company European headquarters, serving as an export base.
- United Kingdom: Exports specialized clinical and rehabilitation equipment.
- France: Exports devices from its domestic medical technology sector.
10. Market Trends
Current Global Trends
- Rising Home Healthcare: Driven by an aging population and cost-containment, demand for user-friendly home-use TENS is surging.
- Integration with Digital Health: TENS units are increasingly pairing with smartphone apps for treatment tracking, reminders, and personalized program adjustments.
- Multi-Modality Devices: Consumers prefer single devices that offer TENS, EMS, and sometimes massage functions.
New Technologies
- Wearable & App-Controlled TENS: Discreet, wire-free devices that can be worn under clothing and controlled via a phone.
- Targeted Pulse Forms: Research into specific waveform signatures for different pain types (neuropathic vs. nociceptive).
- Closed-Loop Systems: Experimental devices that adjust stimulation parameters in real-time based on physiological feedback.
Demand Drivers
- Global Burden of Chronic Pain: Increasing prevalence of conditions like arthritis and low back pain.
- Opioid Crisis: A major push for non-pharmacological pain management alternatives.
- Sports and Fitness Awareness: Growth in usage for muscle recovery and performance.
Future Insights
The market is expected to grow steadily. Future devices will likely be smarter, more connected (IoT), and more personalized, potentially integrating with AI to optimize treatment protocols based on user data. Regulatory focus on software in medical devices (SaMD) will also increase.
11. Training
Required Competency
While simple for home use, professional competency includes:
- Understanding pain physiology and electrotherapy principles.
- Proficiency in anatomical electrode placement for various conditions.
- Ability to select appropriate parameters (frequency, pulse width, duration).
- Knowledge of contraindications and safety protocols.
Common User Errors
- Poor Electrode Placement: Placing electrodes directly on the painful site instead of over related nerve pathways. Solution: Follow anatomical charts or professional guidance.
- Insufficient Conductivity: Using dried-out electrodes or placing them over hairy skin. Solution: Use fresh electrodes, trim hair, and clean skin with alcohol wipes.
- “More is Better” Mentality: Using excessively high intensity or duration. Solution: Use the lowest effective intensity and follow prescribed treatment times (typically 30-60 minutes).
- Impatience: Expecting immediate, permanent cure. Solution: Understand TENS as a management tool; consistent use over sessions is key.
Best-Practice Tips
- Start Low, Go Slow: Begin with low frequency and intensity, gradually increasing to a strong, comfortable tingling (paresthesia).
- Move Electrodes Periodically: To prevent skin irritation and improve efficacy, slightly change pad positions each session.
- Use a Timer: Avoid overuse. Adhere to the treatment time recommended by your therapist.
- Maintain Your Device: Regularly check wires for fraying and keep electrodes fresh.
12. FAQs
1. How long does the pain relief from a TENS unit last?
Relief varies. It can last from minutes to several hours post-treatment. For chronic conditions, regular sessions (e.g., 2-3 times daily) are often recommended for sustained management.
2. Can I become dependent on or tolerant to TENS?
No. TENS is non-addictive. However, the body can sometimes “accommodate” to a fixed setting. Use devices with modulation modes or vary your settings slightly between sessions to prevent this.
3. Why do my electrodes stop sticking?
Skin oils, sweat, and dead skin cells degrade the adhesive. Clean your skin before application and clean reusable electrodes after each use. The gel itself also dries out over time—reusable pads typically last 15-30 uses.
4. Can I use a TENS unit for weight loss or muscle building?
No. TENS is for pain relief and does not cause significant muscle contraction or fat burning. For muscle strengthening, you need an EMS (Electrical Muscle Stimulation) unit, which uses different parameters.
5. Is it safe to use a TENS unit every day?
Yes, for most people, following recommended session durations and guidelines, daily use is safe. Always follow the advice of your healthcare provider.
6. What’s the difference between TENS and an EMS unit?
TENS targets sensory nerves for pain relief with high-frequency, low-intensity pulses. EMS targets motor nerves to cause muscle contractions for rehabilitation, strengthening, or recovery, using lower-frequency, higher-intensity pulses. Many devices now combine both functions.
7. Can I buy a TENS unit without a prescription?
In most countries, including the USA and UK, basic TENS units are available over-the-counter. However, some high-power or insurance-reimbursable models may require a prescription. It is always advisable to consult a professional before first use.
8. Where should I NOT place the electrodes?
Never place electrodes: transversely across the chest, over the front/side of the neck, on the head, over broken/inflamed skin, or directly over the eyes or mouth. When in doubt, consult a placement guide or your therapist.
9. Will TENS interfere with my other medications?
TENS does not interact pharmacologically with medications. However, it can reduce your need for pain medication. Discuss this with your doctor to safely adjust any pain medication regimen.
10. What does it feel like?
When on, you should feel a strong but comfortable tingling, buzzing, or tapping sensation. It should not be painful, burn, or feel like a sharp jab.
13. Conclusion
The TENS unit stands as a cornerstone of non-invasive, drug-free pain management within physiotherapy and beyond. Its efficacy, rooted in established neurophysiological principles like the Gate Control Theory, offers a safe and patient-empowering tool for a wide spectrum of acute and chronic pain conditions. From advanced clinical models to discreet wearable versions, the technology continues to evolve, integrating with digital health trends to provide more personalized care.
Success with TENS hinges on three pillars: appropriate patient selection, correct electrode placement and parameter settings, and a clear understanding of its role as a pain management tool rather than a cure. By adhering to safety guidelines, recognizing contraindications, and procuring quality devices from reputable manufacturers, clinicians and patients alike can harness the significant benefits of this versatile technology. As the global focus on alternative pain management intensifies, the TENS unit is poised to remain an essential and increasingly intelligent component of holistic therapeutic practice.
14. References
- Johnson, M. I., et al. (2019). “The clinical efficacy of transcutaneous electrical nerve stimulation (TENS): A meta-analysis of relevant trials.” Journal of Pain Research.
- Vance, C. G., Dailey, D. L., & Sluka, K. A. (2020). “Using TENS for Pain Control: Update on the State of the Evidence.” Current Physical Medicine and Rehabilitation Reports.
- Food and Drug Administration (FDA). (2023). Code of Federal Regulations, Title 21 – Medical Devices. [FDA Website].
- European Commission. (2017). Regulation (EU) 2017/745 on medical devices (MDR). [EUR-Lex].
- World Health Organization (WHO). (2021). Rehabilitation Competency Framework.
- International Organization for Standardization (ISO). ISO 13485:2016 Medical devices — Quality management systems.
- Grand View Research. (2023). Transcutaneous Electrical Nerve Stimulation (TENS) Devices Market Size, Share & Trends Analysis Report.
- Chartered Society of Physiotherapy (CSP). (2022). Guidance on the Clinical Use of Electrophysical Agents.