1. Definition
What is an Endoloop Ligature Device?
An Endoloop Ligature Device is a single-use, disposable surgical instrument designed for minimally invasive (laparoscopic or endoscopic) procedures. Its primary function is to securely ligate (tie off) the base of a polyp, pedicle, or stump of tissue deep within the body. Think of it as a pre-tied, surgeon-controlled suture loop that can be deployed remotely through a narrow scope channel. It is a critical tool for preventing hemorrhage (bleeding) after the resection (removal) of tissue.
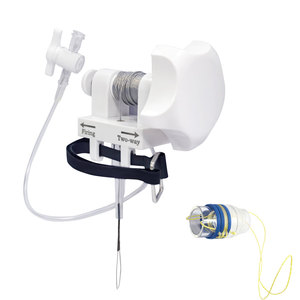
How it Works
The principle is elegantly simple. The device consists of a long, flexible delivery shaft that houses a pre-formed, sterilized suture loop. This loop is held open by a plastic introducer sheath. The surgeon advances the device through the working channel of an endoscope or laparoscope. Once the target structure (e.g., a polyp stalk) is positioned within the open loop, the surgeon retracts the loop by pulling a handle or slider on the proximal end. This action tightens the loop around the base of the tissue, creating a secure ligature. A locking mechanism, often a slip-knot, is then activated to permanently secure the loop, and the delivery system is detached and withdrawn, leaving the ligature in place.
Key Components
- Pre-tied Suture Loop: The core of the device. It is typically made of absorbable or non-absorbable suture material with a pre-formed, self-locking knot.
- Flexible Delivery Shaft/Catheter: A long, thin, and flexible tube that protects the loop and allows it to be delivered through the endoscope.
- Introducer Sheath/Overtube: A clear plastic sheath that keeps the loop open and properly positioned until deployment.
- Control Handle/Slider: The mechanism at the proximal end that the surgeon operates to retract and tighten the loop.
- Knot Pusher (in some designs): A separate component or integrated feature used to advance and secure the knot tightly against the tissue.
2. Uses
Clinical Applications
The Endoloop is predominantly used in:
- Gastroenterology:
- Polypectomy: Ligation of the stalk of large pedunculated polyps in the colon or stomach before or after resection to prevent post-polypectomy bleeding.
- Closure of Perforations: Assisting in the closure of small mucosal defects or perforations.
- Hemostasis: Controlling bleeding from vessels or lesions.
- General Surgery (Laparoscopic):
- Appendectomy: Ligation of the appendix stump during laparoscopic appendectomy.
- Cholecystectomy: Securing the cystic duct or artery in certain techniques.
- Gynecological Surgery: Ligation of pedicles during procedures like ovarian cystectomy or salpingectomy.
- Pediatric Surgery: Useful for ligating structures in confined spaces.
Who Uses It
- Gastroenterologists
- General Surgeons
- Colorectal Surgeons
- Bariatric Surgeons
- Gynecological Surgeons
- Trained Surgical Assistants and Nurses (for preparation and handling)
Departments/Settings
- Endoscopy Suites
- Operating Rooms (OR)
- Ambulatory Surgical Centers (ASCs)
- Day Procedure Units
3. Technical Specs
Typical Specifications
- Working Length: Typically 230 cm for endoscopic use; shorter variants (e.g., 40-60 cm) for laparoscopic use.
- Shaft Diameter: Compatible with standard endoscope channels, commonly 2.8mm or 3.7mm.
- Loop Diameter (when open): Varies, but commonly 20mm, 30mm, or 40mm to accommodate different tissue sizes.
- Suture Material: Absorbable (e.g., Polyglactin/Vicryl) or Non-absorbable (e.g., Polypropylene, Nylon). Absorbable is common as the ligated tissue often sloughs off.
Variants & Sizes
- Loop Size: Available in small, medium, and large diameters.
- Suture Type: Differentiated by absorbable vs. non-absorbable material and suture thickness (e.g., 2-0, 3-0).
- Application-Specific: Some are marketed specifically for appendiceal stump closure or for large polyps.
Materials & Features
- Materials: The shaft is often made of medical-grade plastics like PTFE or FEP for smooth advancement. The suture is USP-standard material.
- Features:
- Slip-Knot Technology: Ensures a secure, self-locking ligation.
- Radiopaque Markers: Allow visualization under fluoroscopy.
- Color-Coded Handles/Packaging: For easy identification of loop size or suture type.
- Low-Profile Design: Minimizes trauma during insertion through the scope.
Models
While often sold as generic “Endoloops,” notable product lines include:
- Olympus: Pre-Cinched Plicator Loops
- Cook Medical: Endoloop® Ligating Device
- Boston Scientific: Resolution® Clip Device (a mechanical alternative, but used for similar indications)
4. Benefits & Risks
Advantages
- Enhanced Safety: Significantly reduces the risk of post-procedural bleeding.
- Minimally Invasive: Enables complex ligation without open surgery.
- Efficiency & Speed: Saves time compared to hand-tying intracorporeal sutures.
- Ease of Use: Simplifies a technically challenging task in a confined space.
- Reliability: Pre-tied, self-locking knots provide consistent and secure ligation.
Limitations
- Single-Use: Disposable nature increases per-procedure costs.
- Limited Reach/Angulation: Can be difficult to deploy effectively on lesions in difficult anatomical locations (e.g., behind folds).
- Loop Slippage: Risk exists if the loop is not placed correctly on a adequate tissue pedicle.
- Tissue Size Constraint: Not suitable for very broad-based or flat lesions.
Safety Concerns & Warnings
- Incomplete Ligation: Can lead to bleeding or leakage.
- Tissue Necrosis: Overtightening or ligating a large mass can cause tissue death.
- Device Failure: Rare, but includes breakage of the suture or failure of the locking mechanism.
- Perforation: The rigid tip of the device can potentially cause trauma if not handled carefully.
Contraindications
- Lesions with a stalk/base thicker than the loop can safely accommodate.
- Suspicion of malignancy at the ligation site (risk of spreading cells).
- Inadequate visualization of the target tissue.
- Known allergy to the suture material.
5. Regulation
As a device that contacts internal tissues and is used to ligate blood vessels, the Endoloop is strictly regulated.
- FDA Class: Typically Class II (moderate to high risk). Regulated under Product Code: GAR.
- EU MDR Class: Typically Class IIa or IIb (Rule 10).
- CDSCO Category (India): Class C (Moderate to High Risk).
- PMDA Notes (Japan): Classified as a “controlled medical device” (Class II).
- ISO/IEC Standards:
- ISO 13485: Quality Management Systems for Medical Devices.
- ISO 10993: Biocompatibility evaluation.
- ISO 11607: Packaging for terminally sterilized medical devices.
- IEC 60601-1: Safety for electrical equipment (if any part is electromechanical).
6. Maintenance
Note: Endoloops are single-use, disposable devices.
Cleaning & Sterilization
Not applicable. The device is supplied sterile (typically via Ethylene Oxide – EtO sterilization) and for single use only. Reprocessing is strictly prohibited.
Reprocessing
Not applicable.
Calibration
Not applicable.
Storage
- Store in a cool, dry place.
- Protect from direct sunlight and moisture.
- Avoid crushing or bending the packaging.
- Observe the “Use By” expiration date on the package.
7. Procurement Guide
How to Select the Device
- Clinical Need: Match the loop size and suture type to your most common procedures (e.g., large loops for big polyps, absorbable sutures for internal use).
- Scope Compatibility: Ensure the shaft diameter fits your endoscope’s working channel.
- Ease of Deployment: Evaluate the handle mechanism for smooth, one-handed operation.
- Cost vs. Value: Balance the unit price against reliability and clinical outcomes.
Quality Factors
- Knot Security: The most critical factor. Look for proven, reliable slip-knot designs.
- Shaft Flexibility: Should be flexible enough to navigate but rigid enough to push without buckling.
- Packaging Integrity: Easy-to-open, sterile barrier packaging.
Certifications
Look for CE Marking (for Europe), FDA 510(k) Clearance (for USA), and other regional regulatory approvals.
Compatibility
Ensure compatibility with your existing endoscope models (Olympus, Pentax, Fujifilm) and their channel sizes.
Typical Pricing Range
Pricing is highly volume-dependent.
- Estimated Range: $50 – $150 per unit.
8. Top 10 Manufacturers (Worldwide)
- Olympus Corporation (Japan) – A global leader in endoscopy with a comprehensive portfolio of endoscopic devices, including high-quality Endoloops.
- Cook Medical LLC (USA) – A pioneer in minimally invasive medical devices, known for its reliable Endoloop® product line.
- Boston Scientific Corporation (USA) – Offers alternative and complementary devices like clips, but is a key player in the hemostasis market.
- Medtronic plc (Ireland) – Through its surgical division, provides a range of laparoscopic and endoscopic instruments.
- Johnson & Johnson (Ethicon Inc.) (USA) – A giant in the surgical suture and stapling market, with relevant ligation products.
- B. Braun Melsungen AG (Germany) – A major European medical device company with a strong presence in surgical sutures and ligation.
- CONMED Corporation (USA) – Specializes in surgical devices, including instruments for laparoscopic procedures.
- Stryker Corporation (USA) – Offers a broad range of surgical equipment, including endoscopic accessories.
- Teleflex Incorporated (USA) – Known for its diverse medical device portfolio, including specialized surgical products.
- Micro-Tech Endoscopy (USA) – A rapidly growing company specializing in innovative endoscopic accessories.
9. Top 10 Exporting Countries (Latest Year)
Based on analysis of trade data for HS Code 901890 – Instruments and appliances used in medical sciences.
- United States: A dominant exporter of high-value, innovative medical devices.
- Germany: A European hub for precision medical engineering.
- Japan: Home to leading endoscopy companies, driving high-value exports.
- Ireland: A major manufacturing and export base for many US-based med-tech companies.
- Mexico: A key manufacturing location for the North American market.
- China: A growing exporter, often competing in the cost-sensitive market segment.
- Netherlands: A central logistics and distribution hub for Europe.
- France: Hosts several major medical device manufacturers.
- United Kingdom: Maintains a strong med-tech sector post-Brexit.
- Switzerland: Known for high-precision, niche medical devices.
10. Market Trends
Current Global Trends
- Rising Procedural Volume: Increasing rates of colon cancer screening (colonoscopies) are directly driving demand.
- Shift to Ambulatory Centers: More procedures are performed in ASCs, which prioritize efficient, single-use devices.
- Cost Containment Pressure: Hospitals are seeking value-based purchasing, balancing cost with clinical efficacy.
New Technologies
- Advanced Hemostatic Clips: Mechanical clips are becoming a competitive alternative for some ligation tasks.
- Hybrid Devices: Combining ligation with electrosurgical cutting in a single device.
- Improved Suture Materials: Development of stronger, more predictable absorbable sutures.
Demand Drivers
- Aging global population.
- Growing awareness and adoption of preventive healthcare.
- Technological advancements in minimally invasive surgery.
Future Insights
The Endoloop will remain a staple tool. Future iterations may feature enhanced deployment mechanisms, integrated sensing to indicate optimal tightness, and bioabsorbable materials with even more tailored degradation profiles.
11. Training
Required Competency
Proficiency in advanced endoscopic or laparoscopic techniques. Surgeons must be able to:
- Navigate the device through a scope.
- Accurately position the loop.
- Judge the appropriate amount of tension to apply.
Common User Errors
- Misplacing the Loop: Failing to get the entire base of the polyp within the loop.
- Incomplete Tightening: Leaving the loop loose, leading to slippage and bleeding.
- Transecting Too Close: Cutting the polyp stalk too close to the ligature, causing it to slip off.
- Over-tightening: Causing immediate transection of the stalk.
Best-Practice Tips
- Position is Key: Ensure the loop is at the very base of the stalk.
- Slow and Steady: Tighten the loop slowly and smoothly under direct vision.
- Check Before You Cut: Confirm the ligature is secure and the tissue is cyanotic (turning blue) before resecting.
- Have a Backup Plan: Always have hemostatic clips or cautery on standby.
12. FAQs
1. Can an Endoloop be used on a sessile (flat) polyp?
No. Endoloops require a defined stalk or pedicle to secure effectively. Flat polyps are typically removed using EMR (Endoscopic Mucosal Resection) or ESD (Endoscopic Submucosal Dissection) techniques.
2. What happens to the Endoloop after the tissue falls off?
If an absorbable suture is used, the body will safely break it down over several weeks. The ligated tissue typically sloughs off and passes naturally.
3. Is there a risk of the loop slipping off?
Yes, if it’s not placed correctly at the narrowest part of the stalk or if it’s not tightened sufficiently. Proper technique is critical to prevent this.
4. Can I use two Endoloops on one polyp?
Yes, for very large or vascular polyps, placing two loops for double ligation is a common and safe practice.
5. What is the difference between an Endoloop and a hemostatic clip?
An Endoloop is a suture that encircles and ligates a stalk. A clip is a mechanical device that pinches and occludes a vessel or tissue. They are often used for different applications or in conjunction.
6. How do I choose between absorbable and non-absorbable suture?
Absorbable is standard for most internal procedures (e.g., polyps, appendix) as the long-term presence of the suture is not required. Non-absorbable may be used in specific cases where permanent ligation is needed.
7. What should I do if the Endoloop breaks during deployment?
Stop the procedure. Carefully retract the broken device without causing trauma. Use an alternative method (e.g., clips, snare cautery) to achieve hemostasis.
8. Why is my Endoloop not advancing through the scope?
Check for scope channel damage or an overly tortuous scope position. Ensure the device is compatible with your scope’s channel size. Never use excessive force.
13. Conclusion
The Endoloop Ligature Device is a cornerstone of modern minimally invasive surgery. Its simple yet effective design provides a reliable method for securing tissue pedicles and preventing complications like post-polypectomy bleeding. While disposable and with specific limitations, its benefits in safety, efficiency, and ease of use make it an indispensable tool in the armamentarium of gastroenterologists and laparoscopic surgeons. Proper selection, adherence to contraindications, and meticulous technique are paramount to leveraging its full potential and ensuring optimal patient outcomes.
14. References
- U.S. Food and Drug Administration (FDA). “Product Classification Database.”
- European Commission. “Medical Device Regulation (MDR) 2017/745.”
- Central Drugs Standard Control Organization (CDSCO). “Medical Device Rules, 2017.”
- Olympus Medical Systems. “Endoloop Instructions for Use.”
- Cook Medical. “Endoloop® Ligating Device Brochure.”
- Tringali, A., et al. (2019). “Endoscopic management of pedunculated colorectal polyps: a review.” Annals of Gastroenterology.
- International Organization for Standardization (ISO). “ISO 13485:2016 Medical devices — Quality management systems.”
- UN Comtrade Database. “Trade Data for HS Code 901890.”