1. Definition
What is a Syringe Pump?
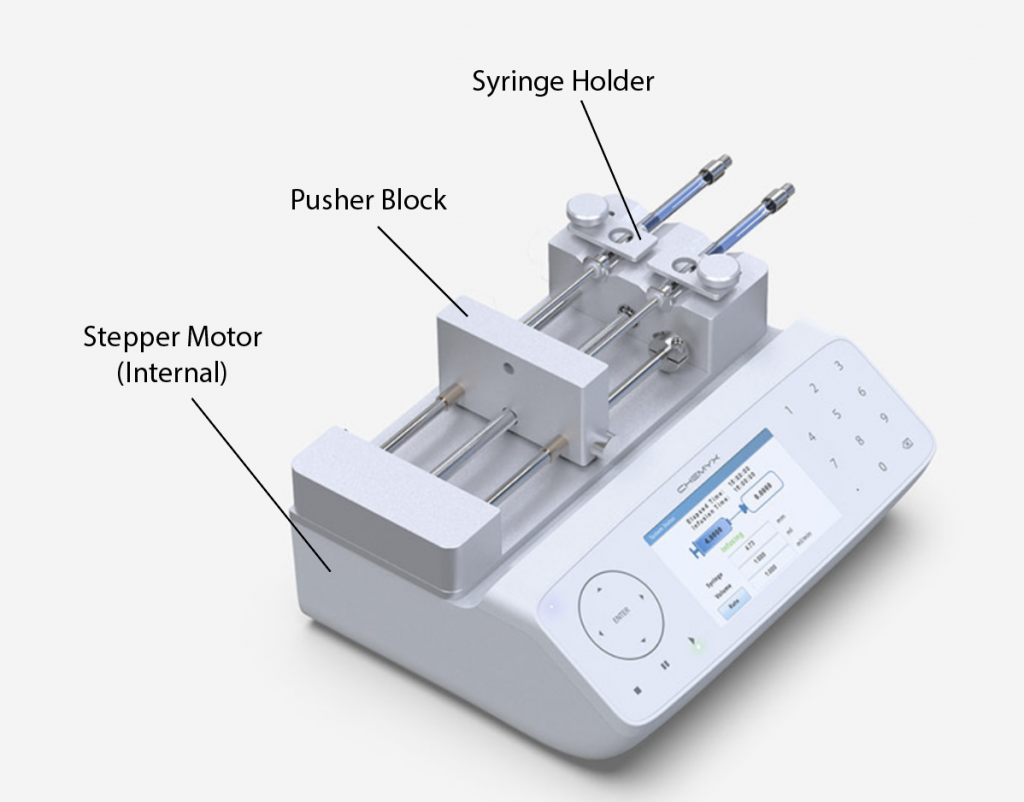
A syringe pump is a precision medical instrument designed to administer small to moderate volumes of fluids (medications, nutrients, or other therapeutic agents) to a patient at a highly accurate, programmed rate over an extended period. It is a critical device for controlled, continuous, or intermittent infusion, where manual administration would be impossible or unsafe due to the required precision and consistency. Think of it as an automated, intelligent hand that pushes the plunger of a syringe with exacting control, measured in microliters (µL) or milliliters (mL) per hour.
How it Works
The fundamental working principle is mechanically simple but electronically sophisticated.
- Loading: A standard medical syringe of a specific size (e.g., 10mL, 50mL) is loaded into a secure holder or cradle on the pump.
- Engagement: The pump’s motor-driven plunger actuator (a pusher block or finger) is advanced to make firm contact with the syringe’s plunger flange.
- Programming: The operator programs the desired infusion parameters via a keypad or touchscreen. This typically includes:
- Infusion Rate: The speed of delivery (e.g., mL/hr, µg/kg/min).
- Volume to be Delivered (VTBD): The total amount of fluid from the syringe to be administered.
- Dose (optional): For drugs, the rate can be calculated based on patient weight and drug concentration.
- Execution: Upon starting, a precision stepper or DC motor rotates, which is translated into linear motion via a lead screw or similar mechanism. This linear motion pushes the plunger actuator forward at a meticulously controlled speed, forcing the fluid out of the syringe and through the connected infusion line into the patient’s IV access.
- Monitoring & Alarms: Sensors and software continuously monitor the infusion. If an issue is detected (e.g., occlusion/blockage, syringe empty, low battery), the pump halts and activates audible and visual alarms to alert the clinician.
Key Components
- Motor: The core driver, usually a stepper motor, provides precise rotational control.
- Lead Screw/Drive Mechanism: Converts the motor’s rotation into the linear motion needed to push the syringe plunger.
- Plunger Actuator/Pusher Block: The component that directly contacts and pushes the syringe plunger.
- Syringe Clamp/Cradle: The holder that secures the syringe barrel firmly in place.
- Control Unit (CPU): The microprocessor that runs the pump’s software, interprets user inputs, controls the motor, and manages alarms.
- User Interface: Typically an LCD screen and keypad or touchscreen for programming and displaying status.
- Power Supply: Battery (for portability/uninterrupted use) and an AC adapter for mains power and charging.
- Alarm System: Buzzer, lights, and on-screen messages for alerts.
- Housing: The durable outer casing, often designed with seals for fluid resistance and easy cleaning.
2. Uses
Clinical Applications
- Critical Care (ICU/NICU): Continuous infusion of vasoactive drugs (e.g., norepinephrine), sedatives (e.g., propofol, midazolam), analgesics (e.g., fentanyl), and insulin. Essential for titrating potent drugs where minute rate changes have significant effects.
- Oncology: Delivery of chemotherapy drugs, antiemetics, and parenteral nutrition, often requiring precise, prolonged infusions.
- Pain Management: Patient-controlled analgesia (PCA) pumps, where patients self-administer bolus doses of pain medication within safe limits set by the clinician.
- Labor & Delivery: Administration of oxytocin for labor induction/augmentation and epidural analgesics.
- Neonatology/Pediatrics: Infusion of medications and fluids for neonates and infants, where extremely low flow rates (0.1 mL/hr) and small total volumes are critical to avoid fluid overload.
- Anesthesiology: Intra-operative infusion of anesthetics, muscle relaxants, and pressor agents.
- Research & Laboratory Use: Precise delivery of reagents, samples, or drugs in pharmaceutical, biomedical, and chemical laboratories.
Who Uses It
- Registered Nurses
- Critical Care Physicians & Intensivists
- Anesthesiologists
- Neonatologists & Pediatricians
- Oncology Nurses
- Research Scientists and Lab Technicians
- Emergency Medical Technicians (in some advanced ambulances)
Departments/Settings
- Intensive Care Units (ICU, CCU, NICU, PICU)
- Oncology/Daycare Chemotherapy Units
- Operating Rooms (OR) & Post-Anesthesia Care Units (PACU)
- Labor & Delivery Suites
- Emergency Departments
- General Medical/Surgical Wards
- Palliative Care Units
- Ambulatory Care Centers
- Biomedical Research Laboratories
3. Technical Specs
Typical Specifications
- Flow Rate Range: Typically from 0.01 mL/hr to 1,800 mL/hr, with high-precision models offering rates as low as 0.001 mL/hr.
- Flow Rate Accuracy: Generally ±1% to ±5%, depending on syringe type, flow rate, and backpressure.
- Syringe Compatibility: Designed for specific syringe brands and sizes (e.g., 5 mL, 10 mL, 20 mL, 30 mL, 50 mL, 60 mL).
- Infusion Modes:
- Continuous Rate
- Intermittent/Bolus
- Weight-Based (µg/kg/min)
- Multi-Step Programming
- PCA (Patient-Controlled Analgesia)
- Occlusion Pressure Limit: User-selectable, often in ranges (e.g., Low: ~500 mmHg, Medium: ~1000 mmHg, High: ~2000 mmHg).
- Power: Rechargeable battery (4-12 hours of operation), AC input (100-240V).
- Display: Backlit LCD or color TFT screen.
- Dimensions & Weight: Varies; portable models are roughly 25 x 15 x 10 cm and weigh 1.5-3 kg.
Variants & Sizes
- Single-Channel Syringe Pump: Manages one syringe. Most common on patient bedside.
- Multi-Channel Syringe Pump: 2, 3, or 4 independently controlled syringe channels in one unit, saving space and power outlets.
- PCA Pumps: Specialized variant with dual-lock security and patient bolus button.
- Ambulatory Pumps: Ultra-portable, lightweight pumps for mobile patients.
- Laboratory Syringe Pumps: Often feature higher precision, RS-232/USB connectivity, and compatibility with non-medical syringes.
Materials & Features
- Materials: Medical-grade ABS/PC plastics, silicone seals, stainless steel or anodized aluminum for drive mechanisms.
- Key Features:
- Drug Libraries: Pre-programmed lists of medications with standardized concentrations, dosing units, and hard/soft limits to reduce programming errors.
- Wireless Connectivity: Integration with hospital EMR (Electronic Medical Record) systems for documentation and pump status monitoring.
- “Keep Vein Open” (KVO) Rate: Automatically switches to a minimal flow rate after primary infusion is complete.
- Pressure Monitoring: Real-time display of line pressure and early occlusion detection.
- Dose Rate Calculation (DRC): Simplifies programming by allowing entry of desired dose and patient weight.
Notable Models (Examples)
- BD Alaris™ Syringe Module (used with Alaris guardrails® system)
- B. Braun Perfusor® Space
- Fresenius Kabi Pilot® Anesthesia / CADD®-Solis
- Smiths Medical Medfusion™ 3500/4000
- Arcomed Chroma/SP6000
- Terumo Terufusion™ Syringe Pump
4. Benefits & Risks
Advantages
- Unmatched Precision & Accuracy: Enables safe administration of high-risk, potent drugs, especially in neonates and critical care.
- Safety: Reduces human calculation and manual injection errors. Integrated drug libraries and alarms add layers of safety.
- Consistency: Maintains a steady plasma concentration of drugs, improving therapeutic efficacy.
- Workflow Efficiency: Frees nursing staff from frequent manual injections or bag changes for small-volume infusions.
- Versatility: Supports a wide range of applications from ultra-slow to relatively fast infusions.
Limitations
- Volume Limit: Constrained by syringe capacity (typically max 50-60mL). Not suitable for large-volume fluid resuscitation.
- Cost: Capital investment and ongoing maintenance costs.
- Complexity: Requires training to program correctly and respond to alarms appropriately.
- Portability: Tethered to the patient and often to an AC outlet, though battery-powered.
Safety Concerns & Warnings
- Occlusion: A blocked line can lead to a dangerous delayed delivery or sudden bolus if pressure is released. Always use the occlusion pressure alarm.
- Programming Errors: Incorrect rate, dose, or concentration entry is the most significant risk. Always double-check settings against the prescription.
- Battery Failure: Can interrupt infusion. Ensure pumps are plugged in when not being transported.
- Syringe Incompatibility: Using an unapproved syringe can lead to grossly inaccurate flow rates. Use only manufacturer-specified syringes.
- Air Embolism: Risk if air is not purged from the syringe and line before connection.
Contraindications
- There are no direct patient contraindications for the device itself. The contraindications always relate to the drug being infused (e.g., allergy, specific patient condition).
- The device is contraindicated for use with non-compatible syringes or fluids that may damage its components.
5. Regulation
Syringe pumps are active therapeutic devices, typically falling under moderate to high-risk classifications globally.
- FDA Class: Class II (moderate to high risk). Requires 510(k) premarket notification to demonstrate substantial equivalence to a legally marketed predicate device.
- EU MDR Class: Class IIa or IIb, depending on intended use. For critical drug delivery (e.g., vasoactive, cytostatic), they are typically Class IIb. Requires a conformity assessment by a Notified Body.
- CDSCO Category (India): Class C (moderate-high risk) under the Medical Device Rules, 2017.
- PMDA (Japan): Classified as Class II controlled medical devices. Requires marketing approval (Shonin) from PMDA.
- ISO/IEC Standards:
- ISO 80601-2-24: Particular requirements for the basic safety and essential performance of infusion pumps and controllers. The paramount standard.
- IEC 60601-1: General requirements for basic safety and essential performance of medical electrical equipment.
- ISO 28620: Medical devices – Non-electrically driven portable infusion devices (covers aspects of mechanical pumps).
6. Maintenance
Cleaning & Sterilization
- Exterior: Clean between patients and periodically with a soft cloth dampened with a mild detergent or hospital-grade disinfectant (e.g., 70% isopropyl alcohol). Avoid spraying liquids directly onto the pump; never immerse it.
- Interior: Not user-serviceable. Requires qualified biomedical engineering (BMET) staff.
- Components: The syringe and infusion set are single-use, sterile items. The pump itself is never sterilized.
Reprocessing
Syringe pumps are non-critical devices (contact only with intact skin). They require cleaning and disinfection between patients, but not sterilization.
Calibration
Critical for accuracy. Must be performed at regular intervals (e.g., annually or per manufacturer’s recommendation) by trained BMET staff using a flow analyzer (e.g., a gravimetric or volumetric analyzer) to verify flow rate accuracy across the entire range.
Storage
- Store in a clean, dry environment.
- Avoid extreme temperatures (refer to manual, typically 10°C to 40°C).
- Store with battery partially charged if not used for extended periods.
- Protect from dust and physical impact.
7. Procurement Guide
How to Select the Device
- Define Clinical Need: ICU, NICU, OR, Oncology? This dictates required flow rate range, accuracy, and features (e.g., drug library for ICU).
- Assess Usability: Interface should be intuitive for staff. Consider screen readability, keypad design, and ease of loading syringes.
- Evaluate Integration: Does it need to connect to your hospital’s EMR or nurse call system?
- Review Safety Features: Prioritize pumps with comprehensive, customizable drug libraries and clear alarm systems.
Quality Factors
- Accuracy & Precision Data: Request independent test reports.
- Reliability & MTBF: Mean Time Between Failures from existing users.
- Ergonomics & Durability: Build quality and user reviews.
- Service & Support: Manufacturer’s local technical support and BMET training availability.
Certifications
- Mandatory: CE Mark (for EU), FDA 510(k) Clearance (for USA), and relevant regional approvals (e.g., CDSCO for India).
- Indicators of Quality: ISO 13485 (Quality Management System) certification of the manufacturing site.
Compatibility
- Syringes: Determine ongoing cost and supply chain for manufacturer-approved syringes.
- Infusion Sets: Check if it works with standard or proprietary tubing.
- Hospital Systems: Compatibility with existing pump management systems or EMRs.
Typical Pricing Range
- Single-Channel Pump: $1,500 – $3,500 per unit.
- Multi-Channel Pump: $3,000 – $6,000 per unit.
- High-Precision/Lab Pumps: $2,000 – $8,000+.
- Note: Pricing is highly variable based on features, region, and purchase volume (bulk contracts).
8. Top 10 Manufacturers (Worldwide)
- Becton, Dickinson and Company (BD) – USA: Global leader via its Alaris™ infusion system, known for its guardrails® safety software.
- B. Braun Melsungen AG – Germany: A powerhouse with the Perfusor® and Space series, renowned for reliability and a vast syringe portfolio.
- Fresenius Kabi – Germany: Major player in infusion therapy, offering the Injectomat®/Pilot® anesthesia pumps and CADD®-Solis ambulatory pumps.
- Smiths Medical (part of ICU Medical) – USA/UK: Known for the Medfusion™ series of syringe pumps, popular in critical care and NICU settings.
- Terumo Corporation – Japan: Leading in Asia with its Terufusion™ series of infusion pumps, including syringe pumps.
- Arcomed AG (Subsidiary of B. Braun) – Switzerland: Specializes in high-precision infusion technology like the Chroma and SP6000 pumps.
- Moog Inc. – USA: Known for high-performance pumps in clinical and research settings, particularly in anesthesia and analgesia.
- Zyno Medical – USA/China: Offers cost-competitive infusion pumps, including syringe pumps, with a focus on emerging markets.
- Mindray – China: A rapidly growing global medtech company with a comprehensive portfolio, including syringe pumps for various care settings.
- Acinoxy (formerly Acin-Flow) – Switzerland: Specializes in high-precision, smart syringe pumps for research and specialized clinical applications.
9. Top 10 Exporting Countries (Latest Year – Based on Trade Data Trends)
(Ranked by estimated export value of HS Code 9018.90 – Other medical instruments)
- Germany: Dominant exporter of high-quality medical devices, home to B. Braun and Fresenius.
- United States: Leading exporter of advanced, digitally integrated pump systems (BD, ICU Medical/Smiths).
- Switzerland: Exports high-precision pumps from Arcomed and others.
- China: Major and growing exporter of mid-range and cost-effective pumps (Mindray, Zyno).
- Japan: Strong exporter in Asia, led by Terumo.
- Ireland: A significant hub for medtech manufacturing and export for multinationals.
- Singapore: Regional distribution and manufacturing hub for Asia-Pacific.
- France: Home to several specialized medical device manufacturers.
- United Kingdom: Historically strong in medtech, with companies like Smiths Medical.
- Netherlands: Key European distribution and manufacturing center.
10. Market Trends
Current Global Trends
- Integration & Interoperability: Pumps are becoming nodes on the Internet of Medical Things (IoMT), feeding data to EMRs and central monitoring stations.
- Shift to Multi-Channel Pumps: To reduce bedside clutter and cost-per-channel in ICU settings.
- Rising Demand in Emerging Markets: Increasing healthcare spending in Asia-Pacific and Latin America drives market growth.
New Technologies
- Smart Drug Libraries & EHR Integration: Reducing errors through auto-population of orders and barcode scanning.
- Advanced Pressure Monitoring: Predictive algorithms to detect occlusions before they become clinically significant.
- Battery & Portability Improvements: Longer-lasting batteries and lighter materials for enhanced ambulatory care.
Demand Drivers
- Rising Chronic Disease Burden: (Cancer, diabetes) requiring long-term, precise drug therapy.
- Expanding Neonatal & Geriatric Care: Populations highly sensitive to infusion errors.
- Focus on Patient Safety: Regulatory pressure to reduce medication errors.
- Growth in Ambulatory & Home Care: Driving demand for portable, user-friendly pumps.
Future Insights
- AI-Powered Predictive Maintenance: Pumps will self-diagnose and alert BMETs before failure.
- Closed-Loop Systems: Pumps will directly receive input from monitors (e.g., glucose sensors for insulin pumps, depth of anesthesia monitors) to automatically titrate infusions.
- Enhanced Cybersecurity: As networked devices, robust security will become a primary design focus.
11. Training
Required Competency
Clinicians must be able to:
- Correctly load and prime the syringe and infusion set.
- Accurately program all infusion parameters (rate, VTBD, dose).
- Navigate the drug library and set appropriate limits.
- Recognize, interpret, and respond appropriately to all alarm conditions.
- Perform basic troubleshooting (check line, connections, power).
Common User Errors
- Wrong Syringe Size Selected in Software: Causes massive flow rate inaccuracy.
- Dose/Rate Calculation Errors: Misplacing decimal points.
- Ignoring or Silencing Alarms: Without addressing the root cause.
- Improper Loading: Syringe not seated firmly, leading to “free flow” or inaccurate delivery.
- Using Incompatible/Non-Approved Syringes or Tubing.
Best-Practice Tips
- ALWAYS perform the “5 Rights” of medication administration before programming.
- Label the syringe clearly with drug, concentration, patient, and rate.
- Use the drug library—it is your primary safety shield.
- Double-check all entries with a second nurse for high-risk infusions.
- Respond to every alarm. Identify and resolve the cause; do not just restart.
- Secure the tubing to prevent tension and accidental dislodgement.
12. FAQs
1. What’s the difference between a syringe pump and a volumetric infusion pump?
Syringe pumps use a motor to push a syringe plunger and are best for small, precise volumes (mL/hr). Volumetric pumps use a rotating mechanism to squeeze standard IV bags/sets and are for larger volumes (100s of mL to Liters per day).
2. Can I use any syringe with my pump?
No. Flow accuracy depends on the syringe’s inner diameter. Use only the syringe brands and sizes specified in the pump’s manual. Using others can result in dangerous under- or over-infusion.
3. The pump is alarming “Occlusion.” What should I do?
- Check if the patient’s IV site is swollen or painful. 2. Check for kinks in the tubing. 3. Ensure all clamps are open. 4. Check if the syringe is empty. Do not simply increase the occlusion pressure limit without finding the cause.
4. How often does the pump need to be serviced?
Preventive Maintenance (PM) and calibration should be done annually or per manufacturer/hospital policy by Biomedical Engineering.
5. Can a syringe pump run without a battery, just on AC power?
Most can, but a functioning battery is critical as a backup during power outages or patient transport. A pump with a dead battery should be taken out of service.
6. What does “KVO” mean?
“Keep Vein Open.” A very low flow rate (e.g., 1-5 mL/hr) the pump switches to after the main infusion finishes, to prevent the IV line from clotting.
7. Is special training required to use a syringe pump?
Yes. Hospital orientation and unit-specific training on the exact model you will use are mandatory for safe operation.
8. How do I program a weight-based drug dose?
You enter the patient’s weight, the drug concentration (e.g., mg/mL), and the desired dose rate (e.g., mcg/kg/min). The pump’s Dose Rate Calculation (DRC) software does the math and sets the flow rate.
9. The screen says “Pump Inoperative” or has a major alarm. What now?
Stop the infusion. Clamp the line manually. Do not attempt to fix it. Switch to a new pump or a manual method as per protocol, and call Biomedical Engineering to remove the faulty pump.
10. How should I dispose of the syringe and tubing after use?
Follow hospital biohazard waste protocol. The syringe and tubing are single-use and go into a sharps or clinical waste container. They are never reused.
13. Conclusion
The syringe pump is an indispensable tool in modern medicine, transforming the delivery of critical and high-risk medications from a manual, error-prone task into a precise, controlled, and safe process. Its value is highest in settings where patient vulnerability is greatest—the NICU, ICU, and OR. Understanding its operation, applications, stringent regulations, and, most importantly, the protocols for its safe use and maintenance is crucial for every healthcare professional involved in infusion therapy. As technology advances, these devices will become smarter and more integrated, further enhancing patient safety and therapeutic outcomes. However, their core purpose will remain the same: to deliver the right drug, at the right dose, to the right patient, with unerring accuracy.
14. References
- International Organization for Standardization. (2020). ISO 80601-2-24:2020 Medical electrical equipment — Part 2-24: Particular requirements for the basic safety and essential performance of infusion pumps and controllers.
- U.S. Food and Drug Administration (FDA). (2023). Infusion Pumps – Total Product Life Cycle. https://www.fda.gov/medical-devices/infusion-pumps
- European Medicines Agency. (2017). Regulation (EU) 2017/745 on medical devices (MDR).
- Institute for Safe Medication Practices (ISMP). (Various). Guidelines for Optimizing Safe Implementation and Use of Smart Infusion Pumps.
- B. Braun, BD, Fresenius Kabi. Operator’s Manuals for Perfusor Space, Alaris Syringe Module, Pilot Anesthesia pumps.
- Grand View Research. (2023). Infusion Pumps Market Size, Share & Trends Analysis Report.
- World Health Organization (WHO). (2010). Technical Specifications for Infusion Devices.
- The Joint Commission. (2020). National Patient Safety Goals on Medication Administration.